Ampicillin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Principen, others[1] |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a685002 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, intravenous |
| ATC code | J01CA01 (WHO) S01AA19 (WHO) QJ51CA01 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% (oral) |
| Protein binding | 15 to 25% |
| Metabolism | 12 to 50% |
| Biological half-life | approx 1 hour |
| Excretion | 75 to 85% renal |
| Identifiers | |
| |
| CAS Number |
69-53-4 |
| PubChem (CID) | 6249 |
| DrugBank |
DB00415 |
| ChemSpider |
6013 |
| UNII |
7C782967RD |
| KEGG |
D00204 |
| ChEBI |
CHEBI:28971 |
| ChEMBL |
CHEMBL174 |
| Chemical and physical data | |
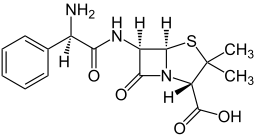
| Formula | C16H19N3O4S |
| Molar mass | 349.41 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Ampicillin is an antibiotic used to prevent and treat a number of bacterial infections, such as respiratory tract infections, urinary tract infections, meningitis, salmonellosis, and endocarditis. It may also be used to prevent group B streptococcal infection in newborns. It is used by mouth, by injection into a muscle, or intravenously.[2] Like all antibiotics, it is not useful for the treatment of viral infections.
Common side effects include rash, nausea, and diarrhea. It should not be used in people who are allergic to penicillin. Serious side effects may include Clostridium difficile colitis or anaphylaxis. While usable in those with kidney problems, the dose may need to be decreased.[2] Its use during pregnancy and breastfeeding appears to be generally safe.[2][3]
Ampicillin was developed in 1961.[4] It is on the World Health Organization's List of Essential Medicines, the most important medications needed in a basic health system.[5] Its wholesale cost in the developing world is between US$0.13 and 1.20 for a vial of the intravenous solution as of 2014.[6] In the United States, it is available as a generic medication and 10 days of treatment cost about $13.[2]
Medical uses
It is active against many Gram-positive and Gram-negative bacteria. Ampicillin was the first 'broad spectrum' penicillin with activity against Gram-positive bacteria including Streptococcus pneumoniae, Streptococcus pyogenes, some isolates of Staphylococcus aureus (but not penicillin-resistant or methicillin-resistant strains), and some Enterococcus. Activity against Gram-negative bacteria includes Neisseria meningitidis, some Haemophilus influenzae, and some of the Enterobacteriaceae. Its spectrum of activity is enhanced by co-administration of sulbactam, a drug that inhibits beta lactamase, an enzyme produced by bacteria to inactivate ampicillin and related antibiotics.[7][8]
It is used for the treatment of infections known to be or highly likely to be caused by these bacteria. These include common respiratory infections including sinusitis, bronchitis, and pharyngitis, as well as otitis media. In combination with vancomycin (which provides coverage of ampicillin-resistant pneumococci), it is effective for the treatment of bacterial meningitis. It is also used for gastrointestinal infections caused by consuming contaminated water or food, such as Salmonella, Shigella, and Listeria.[9]
Ampicillin is a first-line agent for the treatment of infections caused by Enterococci. The bacteria are an important cause of healthcare-associated infections such as endocarditis, meningitis, and catheter-associated urinary tract infections that are typically resistant to other antibiotics.[9]
Side effects
Ampicillin is relatively nontoxic. Its most common side effects include rash, diarrhea, and nausea.[2] In very rare cases, it causes severe side effects such as angioedema, anaphylaxis, and C. difficile colitis.
Mechanism of action
Ampicillin is in the penicillin group of beta-lactam antibiotics and is part of the aminopenicillin family. It is roughly equivalent to amoxicillin in terms of activity.[10]
Ampicillin is able to penetrate Gram-positive and some Gram-negative bacteria. It differs from penicillin G, or benzylpenicillin, only by the presence of an amino group. That amino group helps the drug penetrate the outer membrane of Gram-negative bacteria.
Ampicillin acts as an irreversible inhibitor of the enzyme transpeptidase, which is needed by bacteria to make their cell walls.[10] It inhibits the third and final stage of bacterial cell wall synthesis in binary fission, which ultimately leads to cell lysis; therefore, ampicillin is usually bacteriolytic.[11][10]
History
Ampicillin has been used extensively to treat bacterial infections since 1961.[12] Until the introduction of ampicillin by the British company Beecham, penicillin therapies had only been effective against Gram-positive organisms such as staphylococci and streptococci.[11] Ampicillin (originally branded as 'Penbritin') also demonstrated activity against Gram-negative organisms such as H. influenzae, coliforms, and Proteus spp.[12]
Cost
Its wholesale costs is between US$0.13 and 1.20 for a vial of the intravenous solution as of 2014.[6] In the United States, it is available as a generic medication and 10 days of treatment cost about $13.[2]
See also
- Amoxycillin (p-hydroxy metabolite of ampicillin)
- Pivampicillin (special pro-drug of ampicillin)
- Azlocillin and pirbenicillin (urea and amide made from ampicillin)
References
- ↑ Drugs.com International trade names for Ampicillin Retrieved 14 January 2015
- 1 2 3 4 5 6 "Ampicillin". The American Society of Health-System Pharmacists. Retrieved 1 August 2015.
- ↑ "Ampicillin use while Breastfeeding". March 2015. Retrieved 1 August 2015.
- ↑ Ravina, Enrique (2011). The evolution of drug discovery : from traditional medicines to modern drugs (1 ed.). Weinheim: Wiley-VCH. p. 262. ISBN 9783527326693.
- ↑ "WHO Model List of EssentialMedicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- 1 2 "Ampicillin". International Drug Price Indicator Guide. Retrieved 1 August 2015.
- ↑ Hauser AR (2013). Antibiotic Basics for Clinicians, 2nd ed. Lippincott, Williams, and Wikins, pp. 25–28
- ↑ Akova M (January 2008). "Sulbactam-containing beta-lactamase inhibitor combinations". Clin. Microbiol. Infect. 14 Suppl 1: 185–8. doi:10.1111/j.1469-0691.2007.01847.x. PMID 18154545.
- 1 2 Finberg R, Fingeroth J in Longo DL, Fauci AS, Kasper DL, Hauser SL, Jameson JL, Loscalzo (eds.) (2012) Harrison's Principles of Internal Medicine, 18th ed., McGraw-Hill, Chapter 132.
- 1 2 3 AHFS DRUG INFORMATION 2006 (2006 ed.). American Society of Health-System Pharmacists. 2006.
- 1 2 Petri WA in Brunton LL, Chabner BA, Knollmann BC (eds.) (2011 ) Goodman and Gilman's The Pharmacological Basis of Therapeutics, 12th ed., Chapter 53. McGraw-Hill, New York.
- 1 2 Acred, P; Brown, D. M.; Turner, D. H.; Wilson, M. J. (April 1962). "Pharmacology and chemotherapy of ampicillin--a new broad-spectrum penicillin". Br J Pharmacol Chemother. 18 (2): 356–69. doi:10.1111/j.1476-5381.1962.tb01416.x. PMC 1482127
 . PMID 13859205.
. PMID 13859205.
External links
- Ampicillin bound to proteins in the PDB