Organometallic chemistry

Organometallic chemistry is the study of chemical compounds containing at least one bond between a carbon atom of an organic compound and a metal, including alkaline, alkaline earth, transition metal, and other cases.[1] Moreover, some related compounds such as transition metal hydrides and metal phosphine complexes are often included in discussions of organometallic compounds. The field of organometallic chemistry combines aspects of traditional inorganic and organic chemistry.[2]
Organometallic compounds are widely used both stoichiometrically in research and industrial chemical reactions, as well as in the role of catalysts to increase the rates of such reactions (e.g., as in uses of homogeneous catalysis), where target molecules include polymers, pharmaceuticals, and many other types of practical products.
Organometallic compounds
Organometallic compounds are distinguished by the prefix "organo-" e.g. organopalladium compounds. Examples of such organometallic compounds include all Gilman reagents, which contain lithium and copper. Tetracarbonyl nickel, and ferrocene are examples of organometallic compounds containing transition metals. Other examples include organomagnesium compounds like iodo(methyl)magnesium MeMgI, dimethylmagnesium (Me2Mg), and all Grignard reagents; organolithium compounds such as n-butyllithium (n-BuLi), organozinc compounds such as diethylzinc (Et2Zn) and chloro(ethoxycarbonylmethyl)zinc (ClZnCH2C(=O)OEt); and organocopper compounds such as lithium dimethylcuprate (Li+[CuMe2]−).
The term "metalorganics" usually refers to metal-containing compounds lacking direct metal-carbon bonds but which contain organic ligands. Metal beta-diketonates, alkoxides, and dialkylamides are representative members of this class.
In addition to the traditional metals, lanthanides, actinides, and semimetals, elements such as boron, silicon, arsenic, and selenium are considered to form organometallic compounds, e.g. organoborane compounds such as triethylborane (Et3B).
- Representative Organometallic Compounds
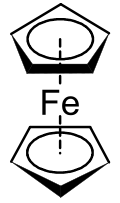 Ferrocene is an archetypal organoiron complex. It is an air-stable, sublimable compound.
Ferrocene is an archetypal organoiron complex. It is an air-stable, sublimable compound. Cobaltocene is a structural analogue of ferrocene, but is highly reactive toward air.
Cobaltocene is a structural analogue of ferrocene, but is highly reactive toward air.P3again.png) Tris(triphenylphosphine)rhodium carbonyl hydride is used in the commercial production of many aldehyde-based fragrances.
Tris(triphenylphosphine)rhodium carbonyl hydride is used in the commercial production of many aldehyde-based fragrances. Zeise's salt is an example of a transition metal alkene complex.
Zeise's salt is an example of a transition metal alkene complex. Trimethylaluminium is an organometallic compound with a bridging methyl group. It is used in the industrial production of some alcohols
Trimethylaluminium is an organometallic compound with a bridging methyl group. It is used in the industrial production of some alcohols
Coordination compounds with organic ligands
Many complexes feature coordination bonds between a metal and organic ligands. The organic ligands often bind the metal through a heteroatom such as oxygen or nitrogen, in which case such compounds are considered coordination compounds. However, if any of the ligands form a direct M-C bond, then complex is usually considered to be organometallic, e.g., [(C6H6)Ru(H2O)3]2+. Furthermore, many lipophilic compounds such as metal acetylacetonates and metal alkoxides are called "metalorganics."
Many organic coordination compounds occur naturally. For example, hemoglobin and myoglobin contain an iron center coordinated to the nitrogen atoms of a porphyrin ring; magnesium is the center of a chlorin ring in chlorophyll. The field of such inorganic compounds is known as bioinorganic chemistry. In contrast to these coordination compounds, methylcobalamin (a form of Vitamin B12), with a cobalt-methyl bond, is a true organometallic complex, one of the few known in biology. This subset of complexes are often discussed within the subfield of bioorganometallic chemistry.[3] Illustrative of the many functions of the B12-dependent enzymes, the MTR enzyme catalyzes the transfer of a methyl group from a nitrogen on N5-methyl-tetrahydrofolate to the sulfur of homocysteine to produce methionine.
The status of compounds in which the canonical anion has a delocalized structure in which the negative charge is shared with an atom more electronegative than carbon, as in enolates, may vary with the nature of the anionic moiety, the metal ion, and possibly the medium; in the absence of direct structural evidence for a carbon–metal bond, such compounds are not considered to be organometallic.
Structure and properties
The metal-carbon bond in organometallic compounds are generally highly covalent. For highly electropositive elements, such as lithium and sodium, the carbon ligand exhibits carbanionic character, but free carbon-based anions are extremely rare, an example being cyanide.
Concepts and techniques
As in other areas of chemistry, electron counting is useful for organizing organometallic chemistry. The 18-electron rule is helpful in predicting the stabilities of metal carbonyls and related compounds. Most organometallic compounds do not however follow the 18e rule. Chemical bonding and reactivity in organometallic compounds is often discussed from the perspective of the isolobal principle.
As well as X-ray diffraction, NMR and infrared spectroscopy are common techniques used to determine structure. The dynamic properties of organometallic compounds is often probed with variable-temperature NMR and chemical kinetics.
Organometallic compounds undergo several important reactions:
- oxidative addition and reductive elimination
- transmetalation
- carbometalation
- hydrometalation
- electron transfer
- beta-hydride elimination
- organometallic substitution reaction
- carbon-hydrogen bond activation
- cyclometalation
- migratory insertion
- nucleophilic abstraction
History
Early developments in organometallic chemistry include Louis Claude Cadet's synthesis of methyl arsenic compounds related to cacodyl, William Christopher Zeise's[4] platinum-ethylene complex,[5] Edward Frankland's discovery of dimethyl zinc, Ludwig Mond's discovery of Ni(CO)4,[1] and Victor Grignard's organomagnesium compounds. The abundant and diverse products from coal and petroleum led to Ziegler-Natta, Fischer-Tropsch, hydroformylation catalysis which employ CO, H2, and alkenes as feedstocks and ligands.
Recognition of organometallic chemistry as a distinct subfield culminated in the Nobel Prizes to Ernst Fischer and Geoffrey Wilkinson for work on metallocenes. In 2005, Yves Chauvin, Robert H. Grubbs and Richard R. Schrock shared the Nobel Prize for metal-catalyzed olefin metathesis.[6]
Organometallic chemistry timeline
- 1760 Louis Claude Cadet de Gassicourt investigates inks based on cobalt salts and isolates cacodyl from cobalt mineral containing arsenic
- 1827 William Christopher Zeise produces Zeise's salt; the first platinum / olefin complex
- 1848 Edward Frankland discovers diethylzinc
- 1863 Charles Friedel and James Crafts prepare organochlorosilanes
- 1890 Ludwig Mond discovers nickel carbonyl
- 1899 Introduction of Grignard reaction
- 1899 John Ulric Nef discovers alkynation using sodium acetylides.
- 1900 Paul Sabatier works on hydrogenation organic compounds with metal catalysts. Hydrogenation of fats kicks off advances in food industry, see margarine
- 1909 Paul Ehrlich introduces Salvarsan for the treatment of syphilis, an early arsenic based organometallic compound
- 1912 Nobel Prize Victor Grignard and Paul Sabatier
- 1930 Henry Gilman works on lithium cuprates, see Gilman reagent
- 1951 Walter Hieber was awarded the Alfred Stock prize for his work with metal carbonyl chemistry.
- 1951 Ferrocene is discovered
- 1963 Nobel prize for Karl Ziegler and Giulio Natta on Ziegler-Natta catalyst
- 1965 Discovery of cyclobutadieneiron tricarbonyl
- 1968 Heck reaction
- 1973 Nobel prize Geoffrey Wilkinson and Ernst Otto Fischer on sandwich compounds
- 1981 Nobel prize Roald Hoffmann and Kenichi Fukui for creation of the Woodward-Hoffman Rules
- 2001 Nobel prize W. S. Knowles, R. Noyori and Karl Barry Sharpless for asymmetric hydrogenation
- 2005 Nobel prize Yves Chauvin, Robert Grubbs, and Richard Schrock on metal-catalyzed alkene metathesis
- 2010 Nobel prize Richard F. Heck, Ei-ichi Negishi, Akira Suzuki for palladium catalyzed cross coupling reactions
Scope
Subspecialty areas of organometallic chemistry include:
- Period 2 elements: organolithium chemistry, organoberyllium chemistry, organoborane chemistry,
- Period 3 elements: organomagnesium chemistry, organoaluminum chemistry, organosilicon chemistry
- Period 4 elements: organotitanium chemistry, organochromium chemistry, organomanganese chemistry organoiron chemistry, organocobalt chemistry organonickel chemistry, organocopper chemistry, organozinc chemistry, organogallium chemistry, organogermanium chemistry
- Period 5 elements: organoruthenium chemistry, organopalladium chemistry, organosilver chemistry, organocadmium chemistry, organoindium chemistry, organotin chemistry
- Period 6 elements: organolanthanide chemistry, organoosmium chemistry, organoiridium chemistry, organoplatinum chemistry, organogold chemistry, organomercury chemistry, organothallium chemistry, organolead chemistry
- Period 7 elements: organouranium chemistry
The following is a presentation of elements of the periodic table with known compounds of carbon with other elements.
| CH | He | ||||||||||||||||
| CLi | CBe | CB | CC | CN | CO | CF | Ne | ||||||||||
| CNa | CMg | CAl | CSi | CP | CS | CCl | CAr | ||||||||||
| CK | CCa | CSc | CTi | CV | CCr | CMn | CFe | CCo | CNi | CCu | CZn | CGa | CGe | CAs | CSe | CBr | CKr |
| CRb | CSr | CY | CZr | CNb | CMo | CTc | CRu | CRh | CPd | CAg | CCd | CIn | CSn | CSb | CTe | CI | CXe |
| CCs | CBa | CHf | CTa | CW | CRe | COs | CIr | CPt | CAu | CHg | CTl | CPb | CBi | CPo | CAt | Rn | |
| Fr | CRa | Rf | Db | CSg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| CLa | CCe | CPr | CNd | CPm | CSm | CEu | CGd | CTb | CDy | CHo | CEr | CTm | CYb | CLu | |||
| Ac | CTh | CPa | CU | CNp | CPu | CAm | CCm | CBk | CCf | CEs | Fm | Md | No | Lr | |||
| Core organic chemistry | Many uses in chemistry |
| Academic research, but no widespread use | Bond unknown |
Industrial applications
Organometallic compounds find wide use in commercial reactions, both as homogeneous catalysis and as stoichiometric reagents For instance, organolithium, organomagnesium, and organoaluminium compounds, examples of which are highly basic and highly reducing, are useful stoichiometrically, but also catalyze many polymerization reactions.[2]
Almost all processes involving carbon monoxide rely on catalysts, notable examples being described as carbonylations.[7] The production of acetic acid from methanol and carbon monoxide is catalyzed via metal carbonyl complexes in the Monsanto process and Cativa process. Most synthetic aldehydes are produced via hydroformylation. The bulk of the synthetic alcohols, at least those larger than ethanol, are produced by hydrogenation of hydroformylation-derived aldehydes. Similarly, the Wacker process is used in the oxidation of ethylene to acetaldehyde.[8]
Almost all industrial processes involving alkene-derived polymers rely on organometallic catalysts. The world's polyethylene and polypropylene are produced via both heterogeneously via Ziegler-Natta catalysis and homogeneously, e.g., via constrained geometry catalysts.[9]
Most processes involving hydrogen rely on metal-based catalysts. Whereas bulk hydrogenations, e.g. margarine production, rely on heterogeneous catalysts, For the production of fine chemicals, such hydrogenations rely on soluble organometallic complexes or involve organometallic intermediates.[10] Organometallic complexes allow these hydrogenations to be effected asymmetrically.

Many semiconductors are produced from trimethylgallium, trimethylindium, trimethylaluminium, and trimethylantimony. These volatile compounds are decomposed along with ammonia, arsine, phosphine and related hydrides on a heated substrate via metalorganic vapor phase epitaxy (MOVPE) process in the production of light-emitting diodes (LEDs).
Environmental concerns
Natural and contaminant organometallic compounds are found in the environment. Some that are remnants of human use, such as organolead and organomercury compounds, are toxicity hazards. Tetraethyllead was prepared for use as a gasoline additive but has fallen into disuse because of lead's toxicity. Its replacements are other organometallic compounds, such as ferrocene and methylcyclopentadienyl manganese tricarbonyl (MMT).[11] The organoarsenic compound roxarsone is a controversial animal feed additive. In 2006, approximately one million kilograms of it were produced in the U.S alone.[12]

See also
References
- 1 2 Crabtree, Robert H. (2009). The Organometallic Chemistry of the Transition Metals (5th ed.). New York, NY: John Wiley and Sons. pp. 2, 560, and passim. ISBN 0470257628. Retrieved 23 May 2016.
- 1 2 Oliveira, José; Elschenbroich, Christoph (2006). Organometallics (3., completely rev. and extended ed.). Weinheim: Wiley-VCH-Verl. ISBN 978-3-527-29390-2.
- ↑ Berg, Jeremy M.; Lippard, Stephen J. (1994). Principles of bioinorganic chemistry ([Pbk. ed.]. ed.). Mill Valley: University Science Books. ISBN 0-935702-73-3.
- ↑ Hunt, L. B. (1984). "The First Organometallic Compounds: William Christopher Zeise and his Platinum Complexes" (PDF). Platinum Metals Rev. 28 (2): 76–83.
- ↑ Zeise, W. C. (1831). "Von der Wirkung zwischen Platinchlorid und Alkohol, und von den dabei entstehenden neuen Substanzen". Annalen der Physik. 97 (4): 497–541. Bibcode:1831AnP....97..497Z. doi:10.1002/andp.18310970402.
- ↑ Dragutan, V.; Dragutan, I.; Balaban, A. T. (2006). "2005 Nobel Prize in Chemistry". Platinum Metals Review. 50 (1): 35–37. doi:10.1595/147106706X94140. ISSN 0032-1400.

- ↑ W. Bertleff; M. Roeper; X. Sava (2005), "Carbonylation", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a05_217
- ↑ Leeuwen, Piet W.N.M. van (2004). Homogeneous catalysis : understanding the art. Dordrecht: Springer. ISBN 978-1-4020-3176-2.
- ↑ Klosin, Jerzy; Fontaine, Philip P.; Figueroa, Ruth (2015). "Development of Group IV Molecular Catalysts for High Temperature Ethylene-α-Olefin Copolymerization Reactions". Accounts of Chemical Research. 48 (7): 2004–2016. doi:10.1021/acs.accounts.5b00065. ISSN 0001-4842.
- ↑ Paul N. Rylander, "Hydrogenation and Dehydrogenation" in Ullmann's Encyclopedia of Industrial Chemistry, Wiley-VCH, Weinheim, 2005. doi:10.1002/14356007.a13 487
- ↑ Seyferth, D. (2003). "The Rise and Fall of Tetraethyllead. 2". Organometallics. 22 (25): 5154–5178. doi:10.1021/om030621b.
- ↑ Hileman, B. (April 9, 2007). "Arsenic in Chicken Production". Chemical and Engineering News. pp. 34–35.
Further reading
- Elschenbroich, Christoph (2016). Organometallics (3rd ed.). New York, NY: John Wiley and Sons. ISBN 3527805141. Retrieved 23 May 2016.
- Clayden, Jonathan ; Greeves, Nick & Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford, UK: Oxford University Press. pp. 132f 182–196, 218ff. 444f, 509f, 656–693 passim, 858, 1009, 1069–1101, 1107–1131 passim. ISBN 0199270295. Retrieved 2 February 2016.
- Crabtree, Robert H. (2009). The Organometallic Chemistry of the Transition Metals (5th ed.). New York, NY: John Wiley and Sons. ISBN 0470257628. Retrieved 23 May 2016.
- Jenkins, Paul R. (1992). Organometallic Reagents in Synthesis. Oxford Chemistry Primers, No. 3. Oxford, UK: Oxford University Press. ISBN 0198556667. ISSN 1367-109X. Retrieved 23 May 2016.
- Pearson, Anthony J. (1985). Metallo-organic Chemistry. New York, NY: John Wiley and Sons. ISBN 0471904465. Retrieved 23 May 2016.
External links
| Wikiquote has quotations related to: Organometallic chemistry |
- MIT OpenCourseWare: Organometallic Chemistry
- Rob Toreki's Organometallic HyperTextbook
- web listing of US chemists who specialize in organometallic chemistry