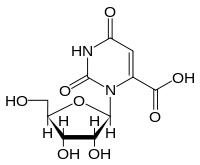
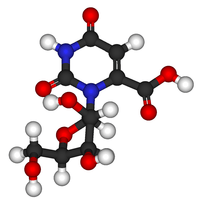
Orotidine
 | |
 | |
| Names | |
|---|---|
| IUPAC names
4-Pyrimidinecarboxylic acid, 1,2,3,6-tetrahydro- 2,6-dioxo-3-beta-D-ribofuranosyl- | |
| Other names
3-Ribofuranosylorotic acid, 6-Carboxyuridine, orotate riboside | |
| Identifiers | |
| 314-50-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 83729 |
| PubChem | 92751 |
| |
| |
| Properties | |
| C10H12N2O8 | |
| Molar mass | 288.213 g/mol |
| Melting point | 200 °C (392 °F; 473 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Orotidine is a nucleoside formed by attaching orotic acid to a ribose ring via a β-N1-glycosidic bond. It is found in bacteria, fungi and plants. It was first isolated in 1951 from the fungus Neurospora by A. Michael Michelson, William Drell, and Herschel K. Mitchell.[1] In humans, orotidine occurs as its 5'-phosphate (orotidylic acid), which is an intermediate in pyrimidine nucleotide biosynthesis (cytidine and uridine) that are found in nucleic acids. Orotidine itself is not a component of nucleic acid. Large amounts of orotidine are excreted in the urine of cancer patients treated with 6-azauridine.
The symbol commonly used for orotidine is O or Ord.
Notes
- ↑ A. Michael Michelson; William Drell; Herschel K. Mitchell (1951). "A new ribose nucleoside from Neurospora: "orotidine"". Proc Natl Acad Sci USA. 37 (7): 396–399. doi:10.1073/pnas.37.7.396. PMC 1063384
 . PMID 14853953.
. PMID 14853953.
This article is issued from Wikipedia - version of the 6/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.