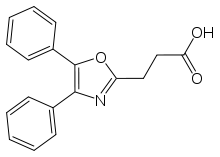
Oxaprozin
 | |
| Clinical data | |
|---|---|
| Trade names | Daypro |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a693002 |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | M01AE12 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 95% |
| Protein binding | 99% |
| Metabolism | Liver—65% oxidation and 35% glucuronic acid conjugation. 5% are active phenolic metabolites. |
| Biological half-life | 54.9 hours |
| Identifiers | |
| |
| CAS Number |
21256-18-8 |
| PubChem (CID) | 4614 |
| IUPHAR/BPS | 7252 |
| DrugBank |
DB00991 |
| ChemSpider |
4453 |
| UNII |
MHJ80W9LRB |
| KEGG |
D00463 |
| ChEBI |
CHEBI:7822 |
| ChEMBL |
CHEMBL1071 |
| ECHA InfoCard | 100.040.254 |
| Chemical and physical data | |
| Formula | C18H15NO3 |
| Molar mass | 293.317 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Oxaprozin, also known as Oxaprozinum, (sold under the names: Daypro, Dayrun, Duraprox) is a non-steroidal anti-inflammatory drug (NSAID),[1] used to relieve the inflammation, swelling, stiffness, and joint pain associated with osteoarthritis and rheumatoid arthritis. Chemically, it is a propionic acid derivative. It is available in 600 mg tablets. Normal adult dosage is 1200 mg daily, not to exceed 1800 mg per day. Safety and efficacy has been established in children over 6 years with juvenile rheumatoid arthritis only, and there is an increased risk of adverse reactions in the elderly population.
Regulatory
FDA Approval
The oxaprozin new drug application (NDA 18-841) was submitted to the FDA on August 10, 1982. The drug was granted an “NDA Day” review on June 15–16, 1992. After Searle agreed to complete seven Phase IV postmarketing studies on October 22, the FDA approved Daypro on October 29, 1992.[2]
Since the approval of Daypro by Searle, other companies have submitted abbreviated new drug applications (ANDAs) to the FDA. Daypro by Searle is listed as the Reference Listed Drug to prove the bioequivalence of the ANDAs. Below is a table listing all of the approved oxaprozin products.
| Company[3] | FDA Approval Date[3] |
|---|---|
| GD Searle | Oct 29, 1992 |
| Apotex Inc | Sep 2, 2004 |
| Dr. Reddy's Labs LTD | Jan 31, 2001 |
| Ivax Sub Teva | May 13, 2002 |
| Sandoz | Jan 31, 2002 |
| Sun Pharm Inds Inc | Jan 3, 2002 |
| Teva | Jul 3, 2002 |
Recalls
Advantage Dose LLC recalled oxaprozin tablets on November 26, 2008. The company was not in conformance with cGMP. (Recall #D-837-2009)[4]
Clinical Studies
Rheumatoid Arthritis
Clinical trials essential to the approval to DAYPRO involved 646 patients. The studies were intended to measure the effect of DAYPRO in regards to the signs and symptoms of rheumatoid arthritis in placebo and active controlled groups. The patients were given single or multiple doses equally 600 to 1800 mg/day. DAYPRO was found to be comparable to 2600 to 3900 mg/day of aspirin. Oxaprozin proved to be more effective and cause fewer adverse reactions than aspirin.
In most of the clinical trials, a 1200 mg dose was given once a day (select patients received up to 1800 mg/day). Some patients responded better to a divided dose. In order to reach its full effect, Daypro was given over the course of several days.[5]
Osteoarthritis
In order to evaluate the effectiveness of Daypro for the signs and symptoms of osteoarthritis, 616 patients participated in active controlled clinical trials against aspirin, piroxicam, and other NSAIDs. Similar to the rheumatoid arthritis clinical trials, patients were given Daypro in both variable and in fixed dosing schedules. Patients received Daypro in either single or divided doses. The variable dosing schedule ranged from 600 to 1200 mg/day and the fixed dosing schedule was set to 1200 mg/day. Oxaprozin was found to be comparable to 2600 to 3200 mg/day doses of aspirin or 20 mg/day doses of piroxicam. The once a day and divided dosing schedules were both effective. Several days of administration were needed for oxaprozin to reach its full effect.[5]
Recent Studies
In 2015, oxaprozin was one of twenty NSAIDs included in a clinical trial to compare the efficacy of NSAIDs in the short-term treatment of ankylosing spondylitis (AS). The NSAIDs were compared by completing randomized controlled trials of NSAIDs in patients with active AS. Efficacy reported at 2–12 weeks and adverse effects were examined. Efficacy was measured by change in pain score and change in the duration of morning stiffness. A total of 26 trials with a total of 3410 participants were completed (58% of the trials had fewer than 50 participants). While all 20 NSAIDs were found to reduce more pain than the placebo, 15 were found to be significantly better. In regards to the decrease of morning stiffness and the likelihood of adverse events, there was no significant difference between NSAIDs. It was concluded that etoricoxib was more effective in reducing pain of AS, however due to small studies and insufficient evidence, no one NSAID could be determined to be the most effective treatment of AS. After etoricoxib, patients taking oxaprozin experienced the least amount of pain with fewer adverse effects than naproxen.[6]
Commercialization
History
Oxaprozin was developed and patented by Wyeth-Ayerst.[2] The US patent 3578671, Oxazoles, was filed November 6, 1967 and published May 11, 1971.[7] Following the filing of the patent, the first description of oxaprozin exhibiting anti-inflammatory properties was outlined in the article Diaryloxazole and diaylthiazolealkanoci acids: two novel series of non-steroidal anti-inflammatory agents. This article was published in Nature in 1968.[8][9] In December 1988, Wyeth-Ayerst licensed the marketing rights for the US, Canada, Puerto Rico, and the Caribbean to Searle.[2]
Daypro became available January 5, 1993. Upon its release, “The Pink Sheet” estimated that the average whole sale price of Searle’s Daypro was $112.30 for 100 (600 mg) tablets.[2] The price was comparable to other prescription NSAIDs.
Other Relevant US Patents
| Patent | Filing Date | Publication Date | Applicant | Title |
|---|---|---|---|---|
| US4532253 | Jul 11, 1984 | Jul 30, 1985 | John Wyeth & Bothers Limited | Oxaprozin calcium salt pharmaceutical compositions |
| US6030643 | May 16, 1997 | Feb 29,2000 | G.D. Searle & Co. | Potassium, sodium and tris oxaprozin salt pharmaceutical formulations |
References
- ↑ Greenblatt DJ, Matlis R, Scavone JM, Blyden GT, Harmatz JS, Shader RI (March 1985). "Oxaprozin pharmacokinetics in the elderly". British Journal of Clinical Pharmacology. 19 (3): 373–8. doi:10.1111/j.1365-2125.1985.tb02656.x. PMC 1463728
 . PMID 3986088.
. PMID 3986088. - 1 2 3 4 The NDA Pipeline 1992. Chevy Chase, MD: F-D-C Reports, Inc. 1992. pp. I–462.
- 1 2 "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 2015-12-02.
- ↑ "FDA Enforcement Report" (PDF). FDA.gov. June 24, 2009. Retrieved Dec 2, 2015.
- 1 2 "DAYPRO Medication Guide" (PDF). accessdata.fda.gov. FDA. 2007. Retrieved 12-2-15. Check date values in:
|access-date=(help) - ↑ Wang, Runsheng (6 Aug 2015). "Comparative efficacy of non-steroidal anti-inflammatory drugs in ankylosing spondylitis: a Bayesian network meta-analysis of clinical trials". Annals of the Rheumatic Diseases. doi:10.1136/annrheumdis-2015-207677.
- 1 2 Oxazoles, retrieved 2015-12-07
- ↑ Brown, K. (July 13, 1968). "Diaryloxazole and Diarylthiazolealkanoic Acids: Two Novel Series of Non-steroidal Anti-inflammatory Agents". Nature. doi:10.1038/219164a0.
- ↑ The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals. Whitehouse Station, NJ: Merck Research Laboratories. 2001.