p-Xylene
 Skeletal formula according to Thiele's resonance concept | |
 Space-filling model | |
| Names | |
|---|---|
| Preferred IUPAC name
1,4-Xylene | |
| Systematic IUPAC name
1,4-Dimethylbenzene | |
| Other names
p-Xylene | |
| Identifiers | |
| 106-42-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27417 |
| ChEMBL | ChEMBL31561 |
| ChemSpider | 7521 |
| ECHA InfoCard | 100.003.088 |
| KEGG | C06756 |
| PubChem | 7809 |
| RTECS number | ZE2625000 |
| UNII | 6WAC1O477V |
| |
| |
| Properties | |
| C8H10 | |
| Molar mass | 106.17 g·mol−1 |
| Appearance | Colorless liquid Colorless crystalline solid |
| Odor | aromatic[1] |
| Density | 0.861 g/mL |
| Melting point | 13.2 °C (55.8 °F; 286.3 K) |
| Boiling point | 138.35 °C (281.03 °F; 411.50 K) |
| insoluble | |
| Solubility in ethanol | very soluble |
| Solubility in diethyl ether | very soluble |
| Vapor pressure | 9 mmHg (20°C)[1] |
| Refractive index (nD) |
1.49582 |
| Viscosity | 0.7385 cP at 0 °C 0.6475 cP at 20 °C |
| 0.00 D [2] | |
| Hazards | |
| Main hazards | Harmful or fatal if swallowed. Vapor harmful. Flammable liquid and vapor. |
| Safety data sheet | See: data page External MSDS |
| R-phrases | R10 R20 R21 R36 R38 |
| S-phrases | S25 |
| NFPA 704 | |
| Flash point | 27 °C (81 °F; 300 K) [3] |
| 528 °C (982 °F; 801 K)[3] | |
| Explosive limits | 1.1%-7.0%[1] |
| 100 ppm[3] (TWA), 150 ppm[3] (STEL) | |
| Lethal dose or concentration (LD, LC): | |
| LC50 (median concentration) |
4550 ppm (rat, 4 hr)[4] |
| LCLo (lowest published) |
3401 ppm (mouse)[4] |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
TWA 100 ppm (435 mg/m3)[1] |
| REL (Recommended) |
TWA 100 ppm (435 mg/m3) ST 150 ppm (655 mg/m3)[1] |
| IDLH (Immediate danger) |
900 ppm[1] |
| Related compounds | |
| Related aromatic hydrocarbons |
benzene toluene o-xylene m-xylene |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
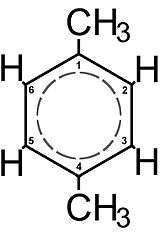
p-Xylene is an aromatic hydrocarbon. It is one of the three isomers of dimethylbenzene known collectively as xylenes. The p- stands for para-, implying that the two methyl groups in p-Xylene occupy the diametrically opposite substituent positions 1 and 4, as illustrated here in the schematic representation. It is in the positions of the two methyl groups, their arene substitution pattern, that it differs from the other isomers, o-xylene and m-xylene. Each of the three isomers has two methyl substituents attached to the benzene ring; ignoring the substitution patterns they therefore all have the same chemical formula C6H4(CH3)2.
Like all xylene isomers, p-xylene is dangerously flammable.
Nomenclature and structure
For historical reasons various sources refer to p-xylene as any of: 1,4-dimethylbenzene, p-dimethylbenzene; p-xylol; 1,4-xylene; p-methyltoluene; para-sylene; chromar; scintillar; 4-methyltoluene; NSC 72419; or 1,4-dimethyl-benzene.[5]
According to convenience, the chemical formula may be represented as C8H10 or C6H4(CH3)2.[6][7][8]
Physical properties
p-Xylene is a colorless, flammable liquid practically insoluble in water. The boiling point is 138.35 °C (281 °F) and the melting point 13.2 °C (56 °F). Its specific gravity is 0.86.
p-Xylene is highly flammable, with a flash point of 27°C.
The odor threshold of p-xylene is 0.62 parts per million (ppm).[6]
Production
The production of p-xylene is industrially significant world-wide, with annual demand estimated at 37 million tons in 2014, and still on the increase.[9]
p-Xylene is produced by catalytic reforming of petroleum naphtha as part of the BTX aromatics (benzene, toluene and the xylene isomers) extracted from the catalytic reformate. The p-xylene is then separated out in a series of distillation, adsorption or crystallization and reaction processes from the m-xylene, o-xylene and ethylbenzene. Its melting point is the highest among this series of isomers, but simple crystallization does not allow easy purification due to the formation of eutectic mixtures.
Such separation procedures are major cost factors in the production of p-xylene, and the search for alternative methods continues. For example, a reverse-osmosis technique has now been proposed to improve various aspects of the processes. [10]
Industrial applications
p-Xylene is an important chemical feedstock. Among other industrial applications, it is a raw material in the large scale synthesis of various polymers. In particular it is a component in the production of terephthalic acid for polyesters such as polyethylene terephthalate. It also may be polymerised directly to produce parylene.
Exposure to p-xylene
Inhalation
Inhaling p-xylene can cause dizziness, headache, drowsiness, and nausea. If exposure through inhalation occurs, first aid includes fresh air, rest and possible medical attention. Through the use of ventilation or breathing protection, exposure to p-xylene through inhalation can be prevented.[7]
Skin
Exposure of p-xylene through the skin can cause dry skin and redness. If skin exposure occurs, first aid includes rinsing and then washing the affected area with soap and water as well as removing any contaminated clothing and thoroughly cleaning and drying before reuse. Exposure can be prevented through the use of protective gloves.[7]
Eyes
Exposure of p-xylene to eyes can cause redness and pain. If eyes are exposed, first aid includes rinsing of the eyes with water for several minutes, removal of contact lenses if applicable, and medical attention. Eye exposure can be prevented through the use of safety glasses or safety goggles.[7]
Ingestion
Ingestion of p-xylene can result in a burning sensation, abdominal pain, dizziness, drowsiness, headache, and nausea. If p-xylene is ingested one's mouth should be rinsed and vomiting should not be induced. Further medical attention should be sought. Ingestion can be prevented by not eating, drinking, or smoking when working with p-xylene.[7]
Short-term exposure
p-Xylene can cause issues with the central nervous system and if swallowed could cause chemical pneumonitis when breathed into the lungs.[7]
Long-term exposure
Liquid p-xylene exposure to the skin over long periods of time can remove the fat from the skin. The substance may also have effects on the central nervous system . Exposure can enhance hearing loss caused by noise exposure. Animal tests suggest that this substance could cause damage to human development and reproductive systems.[7]
Toxicity
Overexposure of p-xylene in humans can cause headache, fatigue, dizziness, listlessness, confusion, irritability, gastrointestinal disturbances including nausea and loss of appetite, flushing of the face, and a feeling of increased body heat. p-Xylene vapor exposure over the recommended exposure limit of 100 parts per million (ppm) can cause irritation to eye, nose, and throat and possible chest tightening and an abnormal gait.[8]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0670". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Perry's Handbook of Chemical Engineers
- 1 2 3 4 "p-Xylene". International Chemical Safety Cards. ICSC/NIOSH. July 1, 2014.
- 1 2 "Xylenes". Immediately Dangerous to Life and Health. National Institute for Occupational Safety and Health (NIOSH).
- ↑ "p-xylene". NIST. Retrieved 21 February 2013.
- 1 2 "p-Xylene MSDS". ScienceLab.com.
- 1 2 3 4 5 6 7 "para-Xylene". National Institute of Occupational Safety and Health. Retrieved 12 February 2013.
- 1 2 "MATERIAL SAFETY DATA SHEET PARA-XYLENE". Amoco. Retrieved 13 February 2013.
- ↑ Nature 532,435–437 (28 April 2016) doi:10.1038/532435a
- ↑
