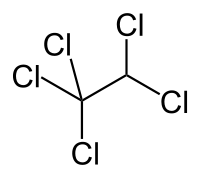
Pentachloroethane
 | |
| Names | |
|---|---|
| IUPAC name
1,1,1,2,2-pentachloroethane | |
| Identifiers | |
| 76-01-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:76287 |
| ChemSpider | 6179 |
| ECHA InfoCard | 100.000.842 |
| PubChem | 6419 |
| |
| |
| Properties | |
| C2HCl5 | |
| Molar mass | 202.09 g mol−1 |
| Appearance | Colorless liquid |
| Odor | Sweetish, chloroform-like |
| Density | 1.68 g cm−3 |
| Melting point | −29 °C (−20 °F; 244 K) |
| Boiling point | 162 °C (324 °F; 435 K) |
| 0.05% (20°C)[1] | |
| Vapor pressure | 3 mmHg (20°C)[1] |
| Hazards | |
| EU classification (DSD) |
|
| R-phrases | R11, R20, R23/24/25, R36/38, R39, R40, R48, R51 |
| S-phrases | S23, S26, S36/37, S45, S61 |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
Handle with care in the workplace[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Pentachloroethane is a non-flammable but toxic chemical compound of chlorine, hydrogen, and carbon. It is used as a solvent for oil and grease, in metal cleaning, and in the separation of coal from impurities.
References
External links
This article is issued from Wikipedia - version of the 5/14/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.