Peripatric speciation
| Part of a series on |
| Evolutionary biology |
|---|
 |
|
History of evolutionary theory |
|
Fields and applications
|
|
Peripatric and peripatry are terms from biogeography, referring to organisms whose ranges are closely adjacent but do not overlap, being separated where these organisms do not occur – for example on an oceanic island compared to the mainland. Such organisms are usually closely related (e.g. sister species), their distribution being the result of peripatric speciation.
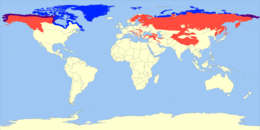

The blue shading indicates the range of the polar bear. The purple shading indicates areas where both live. This is an example of peripatric speciation because polar bears' ancestors moved to a new ecological niche on the periphery of the brown bears' original habitat.]]
Peripatric speciation is a form of speciation, the formation of new species through evolution. In this form, new species are formed in isolated peripheral populations; this is similar to allopatric speciation in that populations are isolated and prevented from exchanging genes. However, peripatric speciation, unlike allopatric speciation, proposes that one of the populations is much smaller than the other. One possible consequence of peripatric speciation is that a geographically widespread ancestral species becomes paraphyletic, thereby becoming a paraspecies. The concept of a paraspecies is therefore a logical consequence of the Evolutionary Species Concept, by which one species give rise to a daughter species. The evolution of the polar bear from the brown bear is a well-documented example of a living species that gave rise to another living species through the evolution of a population located at the margin of the ancestral species' range.[1]
Peripatric speciation was originally proposed by Ernst Mayr, and is related to the founder effect, because small living populations may undergo selection bottlenecks.[2] The founder-effect is based on models that suggest peripatric speciation can occur by the interaction of selection and genetic drift;[3] of which is often proposed to play a significant role in peripatric speciation.[4]
Theoretical framework
Theoretical models of peripatric speciation are identical to models of vicariance (allopatry); however, the nature of dispersal and colonization into novel environments may give rise to rapid speciation events.[3] In addition, individuals colonizing a new habitat likely contain only a small "sample" of the genetic variation of the original population; thus promoting divergence due to selective pressures, and possibly leading to the rapid fixation of an allele within the descendant population. This gives rise to the potential for genetic incompatibilities to evolve.[3]
Laboratory evidence
Peripatric speciation has been researched in both laboratory studies and nature. Coyne and Orr in Speciation suggest that most laboratory studies of allopatric speciation are also examples of peripatric speciation due to their small population sizes and the inevitable divergent selection that they undergo.[3]
Much of the laboratory research concerning peripatry is inextricably linked to founder-effect research. Coyne and Orr conclude that selection's role in speciation is well established, whereas genetic drift's role is unsupported by experimental and field data—suggesting that founder-effect speciation does not occur.[5] Nevertheless, a great deal of research has been conducted on the matter, and one study conducted involving bottleneck populations of Drosophila pseudoobscura found evidence of isolation after a single bottleneck.[6][7]
Observational evidence
Island species provide direct evidence of speciation occurring peripatrically in such that, "the presence of endemic species on oceanic islands whose closest relatives inhabit a nearby continent" must have originated by a colonization event.[3]
Drosophila species on the Hawaiian archipelago have helped researchers understand speciation processes in great detail. It is well established that Drosophila has undergone an adaptive radiation into hundreds of endemic species on the Hawaiian island chain;[3] originating from a single common ancestor (supported from molecular analysis).[8] Studies consistently find that colonization of each island occurred from older to younger islands, speciating peripatrically at least fifty percent of the time.[3] In conjunction with Drosophila, Hawaiian lobeliads (Cyanea) have also undergone an adaptive radiation, with upwards of twenty-seven percent of extant species arising after new island colonization—exemplifying peripatric speciation—once again, occurring in the old-to-young direction.[9][10]
Several other endemic species in Hawaii also provide evidence of peripatric speciation such as the endemic flightless crickets (Laupala). Shaw estimated that, "17 species out of 36 well-studied cases of speciation were peripatric".[3][11] Gillespie and Croom also found evidence of peripatry in Hawaiian Tetragnatha spiders.[12]
Coyne and Orr contend that evidence of peripatry arising on continents is far more difficult to detect due to the possibility of vicarient explanations being equally likely. However, studies concerning the Californian plant species Clarkia biloba and C. biloba strongly suggest a peripatric origin.[13] In addition, a great deal of research had been conducted on several species of land snails involving chirality that suggests peripatry (with some authors noting other possible interpretations).[3]
References
- ↑ Edwards CJ, Suchard MA, Lemey P, et al. (August 2011). "Ancient Hybridization and an Irish Origin for the Modern Polar Bear Matriline". Current Biology. 21: 1251–1258. doi:10.1016/j.cub.2011.05.058. PMID 21737280.
- ↑ Provine WB (1 July 2004). "Ernst Mayr: Genetics and speciation". Genetics. 167 (3): 1041–6. PMC 1470966
 . PMID 15280221.
. PMID 15280221. - 1 2 3 4 5 6 7 8 9 Jerry A Coyne and H. Allen Orr (2004), Speciation, Sinauer Associates, pp. 105–111, ISBN 0-87893-091-4
- ↑ Templeton AR (1 April 1980). "The theory of speciation via the founder principle". Genetics. 94 (4): 1011–38. PMC 1214177
 . PMID 6777243.
. PMID 6777243. - ↑ Jerry A Coyne and H. Allen Orr (2004), Speciation, Sinauer Associates, p. 410, ISBN 0-87893-091-4
- ↑ Jeffrey R. Powell (1978), "The Founder-Flush Speciation Theory: An Experimental Approach", Evolution, 32 (3): 465–474
- ↑ Diane M. B. Dodd and Jeffrey R. Powell (1985), "Founder-Flush Speciation: An Update of Experimental Results with Drosophila", Evolution, 39 (6): 1388–1392
- ↑ DeSalle R. (1995). Molecular approaches to biogeographic analysis of Hawaiian Drosophilidae. Pp. 72-89 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ Givnish, T. J. (1998). Adaptive plant evolution on islands: classical patterns, molecular data, new insights. Evolution on islands, 281, 304.
- ↑ Givnish, T. J., et al. (1995). Molecular evolution, adaptive radiation, and geographic speciation in Cyanea (Campanulaceae, Lobeliodeae). Pp. 259-301 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ Kerry L. Shaw (2002), "Conflict between nuclear and mitochondrial DNA phylogenies of a recent species radiation: What mtDNA reveals and conceals about modes of speciation in Hawaiian crickets", PNAS, 99 (25)
- ↑ Gillespie, R. G. and H. B. Croom (1995). Comparison of speciation mechanisms in web-building and non-web-building groups within a lineage of spiders. Pp. 121-146 in W.L. Wagner and V.A. Funk (eds.) Hawaiian Biogeography: Evolution on a Hot-Spot Archipeligo. Smithsonian Institution Press, Washington DC.
- ↑ H. Lewis and M. R. Roberts (1956), "The origin of Clarkia lingulata", Evolution, 10: 126–138