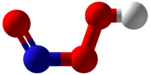
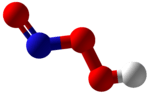
Peroxynitrous acid
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Peroxynitrous acid | |
| Systematic IUPAC name | |
| Identifiers | |
| 14691-52-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:25942 |
| ChEMBL | ChEMBL1794794 |
| ChemSpider | 109951 |
| 49207 | |
| MeSH | Peroxynitrous+Acid |
| PubChem | 123349 |
| |
| |
| Properties | |
| NHO 3 | |
| Molar mass | 63.0128 g mol−1 |
| Related compounds | |
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Peroxynitrous acid (HNO3) is a reactive nitrogen-containing species (RNS). It is the conjugate acid of peroxynitrite (ONOO−). It has a pKa of ~6.8. It is formed in vivo from the diffusion-controlled reaction of nitrogen monoxide (•NO) and superoxide (O•−
2). It isomerises with k = 1.2 s−1, a process whereby up to 5% of hydroxyl and nitrogen dioxide radicals may be formed. It oxidises and nitrates aromatic compounds in low yield. The mechanism may involve a complex between the aromatic compound and ONOOH, and a transition from the cis- to the trans-configuration of ONOOH.[3] Peroxynitrous acid is also important in atmospheric chemistry.
It is an isomer of nitric acid.
References
- ↑ N.Connelly and T. Damhus, IUPAC. Nomenclature of Inorganic Chemistry, RSC Publishing, Cambridge, 2005
- ↑ "Peroxynitrous Acid - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 16 September 2004. Identification and Related Records. Retrieved 11 April 2012.
- ↑ W. H. Koppenol, P. L. Bounds, T. Nauser, R. Kissner, H. Rüegger, "Peroxynitrous acid: controversy and consensus surrounding an enigmatic oxidant", Dalton Trans., 2012, 41, 13779–13787.
This article is issued from Wikipedia - version of the 9/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.