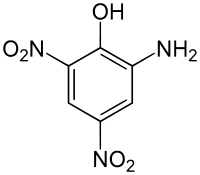
Picramic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Amino-4,6-dinitrophenol | |
| Identifiers | |
| 96-91-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4103087 |
| ECHA InfoCard | 100.002.314 |
| EC Number | 202-544-6 |
| PubChem | 4921319 |
| |
| |
| Properties | |
| C6H5N3O5 | |
| Molar mass | 199.12 g/mol |
| Appearance | Brown paste |
| Density | 1.749 g/cm3 |
| Melting point | 169 °C (336 °F; 442 K) |
| Boiling point | 386.3 °C (727.3 °F; 659.5 K) |
| Hazards | |
| R-phrases | 2, 4, 23/24/25 |
| S-phrases | 28, 35, 37, 45 |
| Flash point | 187.5 °C (369.5 °F; 460.6 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Picramic acid, also known as 2-amino-4,6-dinitrophenol,[1] is an acid obtained by neutralizing an alcoholic solution of picric acid with ammonium hydroxide. Hydrogen sulfide is then added to the resulting solution, which turns red, yielding sulfur and red crystals. These are the ammonium salts of picramic acid, from which it can be extracted using acetic acid.[2]
Picramic acid is explosive and very toxic. It has a bitter taste.[3]
References
This article is issued from Wikipedia - version of the 11/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.