Polyporic acid
 | |
| Names | |
|---|---|
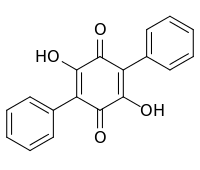
| IUPAC name
2,5-Dihydroxy-3,6-diphenylcyclohexa-2,5-diene-1,4-dione | |
| Other names
Polyporin; Orygameic acid | |
| Identifiers | |
| 548-59-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 10587 |
| MeSH | C118527 |
| PubChem | 11056 |
| |
| |
| Properties | |
| C18H12O4 | |
| Molar mass | 292.29 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Polyporic acid is a terphenyl benzoquinone compound first identified by German chemist Stahlschmidt from a mycelial culture of the fungus species Hapalopilus nidulans in 1877.[1][2] This chemical, present at 20–40% of the fresh weight of the fruit bodies,[3] inhibits the enzyme dihydroorotate dehydrogenase.[4] It is found in other mushrooms, but in much lower amounts.[4] Polyporic acid has some antifungal[5] and antibacterial activity.[6] It has been shown to be an intermediate in the biosynthesis of allantofuranone, a gamma-lactone antibiotic from the black rot fungus Allantophomopsis lycopodina.[7]
References
- ↑ Stahlschmidt C. (1877). "Ueber eine neue in der Natur vorkommende organische Säure" [A new naturally occurring organic acid]. Justus Liebigs Annalen der Chemie. 187 (2–3): 177–197. doi:10.1002/jlac.18771870204.
- ↑ Spatafora C, Calì V, Tringali C (2003). "Polyhydroxy-p-terphenyls and related p-terphenylquinones from fungi: overview and biological properties". Studies in Natural Products Chemistry. 29 (J): 263–307. doi:10.1016/S1572-5995(03)80009-1.
- ↑ Räisänen R. (2009). "Dyes from lichens and mushrooms". In Bechtold T, Mussak R. Handbook of Natural Colorants. Chichester, UK: John Wiley & Sons. p. 192. ISBN 978-0-470-74496-3.
- 1 2 Kraft J, Bauer S, Keilhoff G, Miersch J, Wend D, Riemann D, Hirschelmann R, Holzhausen HJ, Langner J (1998). "Biological effects of the dihydroorotate dehydrogenase inhibitor polyporic acid, a toxic constituent of the mushroom Hapalopilus rutilans, in rats and humans". Archives of Toxicology. 72 (11): 711–721. doi:10.1007/s002040050565. PMID 9879809.
- ↑ Brewer D, Maass WS, Taylor A (1977). "The effect on fungal growth of some 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology. 23 (7): 845–51. doi:10.1139/m77-126. PMID 884625.
- ↑ Brewer D, Jen WC, Jones GA, Taylor A (1984). "The antibacterial activity of some naturally occurring 2,5-dihydroxy-1,4-benzoquinones". Canadian Journal of Microbiology. 30 (8): 1068–1092. doi:10.1139/m84-166. PMID 6541963.
- ↑ Schüffler A, Liermann JC, Opatz T, Anke T (2011). "Elucidation of the biosynthesis and degradation of allantofuranone by isotopic labelling and fermentation of modified precursors". Chembiochem. 12 (1): 148–154. doi:10.1002/cbic.201000448.
This article is issued from Wikipedia - version of the 10/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.