Polythionic acid
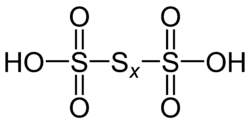
Polythionic acid is an oxoacid which has a straight chain of sulfur atoms and has the chemical formula H2SnO6 (n > 2). Trithionic acid (H2S3O6), tetrathionic acid (H2S4O6) are simple examples. The compounds of n < 80 are expected to exist, and those of n < 20 have already been synthesized. Dithionic acid (H2S2O6) does not belong to polythionic acid due to the difference in the property.
Nomenclature
All polythionates anion contains chains of sulfur atoms attached to the terminal SO3H-groups. Names of polithionic acids are determined by the number of atoms in the chain of sulfur atoms:
- H
2S
2O
6 - dithionic acid - H
2S
3O
6 - trithionic acid - H
2S
4O
6 - tetrathionic acid - H
2S
5O
6 - pentathionic acid, etc.
History
Numerous acids and salts of this group have a venerable history, and chemistry systems, where they exist, dates back to the studies John Dalton devoted to the behavior of H
2S in aqueous solutions of SO
2 (1808). This solution now has the name of Heinrich Wilhelm Ferdinand Wackenroder, who conducted a systematic study (1846). Over the next 60–80 years, numerous studies have shown the presence of ions, in particular tetrathionate and pentathionate anion (S4O62− and S5O62−, respectively).
Preparation and Properties
In the last few decades in the work of Schmidt and other scientists in Germany, a new idea formed: as H
2S can react with SO
3 or HSO
3Cl, forming thiosulfuric acid H
2S
2O
3, as the analogous reaction with H
2S
2 forms disulfonmonosulfonic acid H
2S
2SO
3H; similarly polysulfanes H2Sn (n = 2-6) give HSnSO3H. Reactions from both ends of the polysulfane chain lead to the formation of polysulfondisulfonic acid HO3SSnSO3H.
There are many known methods for the synthesis of these acids, but the reaction mechanism is unclear because of the large number of simultaneously occurring and competing reactions such as redox, chain transfer, and disproportionation. Typical examples are:
- Interaction between hydrogen sulfide and sulfur dioxide. This yields a complex mixture of various oxyacids of sulfur of different structures, called Wackenroder solution.
- H2S + H2SO3 → H2S2O2 + H2O
- H2S2O2 + 2H2SO3 → H2S4O6 + 2H2O
- H2S4O6 + H2SO3 → H2S3O6 + H2S2O3
- Reactions of sulfur halides with HSO3− or HS2O3−, for example :
- SCl2 + 2HSO3− → [O3SSSO 3]2− + 2HCl
- S2Cl2 + 2HSO3− → [O 3SS2SO3]2− + 2HCl
- SCl2 + 2HS2O3− → [O3SS3SO3]2− + 2HCl
- The oxidation of thiosulfate with mild oxidizing agents such as I2, Cu2+, S2O82−, H2O2, MnO2.
- Various special methods of synthesis.
Dithionate ion is obtained by oxidation of aqueous sulfur dioxide in a powder suspension of manganese or iron oxide (MnO2, Fe2O3):
- MnO2 + 2SO2 → MnS2O6
Trithionate ion synthesized by oxidation of thiosulfate ions with hydrogen peroxide:
- 2S2O32− + 4H2O2 → S3O62− + SO42− + 4H2O
Tetrathionate ion can be obtained by oxidation of thiosulfate ion with iodine (reaction is used in iodometry):
- S2O32− + I2 → S4O62− + 2I−
Pentathionate ion is obtained by the action of SCl2 on the thiosulfate ion and Wackenroder solution by adding to it potassium acetate. Initially prismatic crystals of potassium tetrathionate fall, then lamellar crystals of potassium pentathionate, from which the influence of tartaric acid makes an aqueous solution of pentathionic acid.
For potassium hexathionate K2S6O6 the best synthesis action is combining KNO2 and K2S2O3 in concentrated HCl at low temperatures.
Anhydrous polythionic acids can be formed in diethyl ether solution by the following three general ways:
- HSnSO3H + SO3 → H2Sn+2O6 (n = 1, 2 … 8)
- H2Sn + 2SO3 → H2Sn+2O6 (n = 1, 2 … 8)
- 2HSnSO3H + I2 → H2S2n+2O6 + 2HI (n = 1, 2 … 6)
More complex polythionates, with the number of sulfur atoms being 23, form by reacting thiosulfate with SCl2 and S2Cl2.
Polythionic acids with a small number of sulfur atoms in the chain (x = 3 - 6) are the most stable. Polythionic acids are stable only in aqueous solutions, and are rapidly destroyed at higher concentrations with the release of sulfur, sulfur dioxide and - sometimes - sulfuric acid. Acid salts of polythionic acids do not exist. Polythionate ions are significantly more stable than the corresponding acids.
Under the action of oxidants (potassium permanganate, potassium dichromate) polythionic acids and their salts are oxidized to sulfate, and the interaction with strong reducing agents (amalgam of sodium) converts them into sulfites and dithionites.
Occurrence
Polythionic acids are often found in crater lakes. There are various kinds of ions containing sulfur atoms derived by hydrogen sulfide and they make the strongly acidic conditions. It is observed that polythionates in crater lakes are drastically decreased before an eruption occurs.[1] The phenomenon may be useful to predict volcanic activity.
References
- ↑ Takano, B. (1987). "Correlation of Volcanic Activity with Sulfur Oxyanion Speciation in a Crater Lake". Science. 235 (4796): 1633–1635. doi:10.1126/science.235.4796.1633.
- Волынский Н. П., Тиосерная кислота. Политионаты. Реакция Вакенродера. - М.: Наука, 1971. - 80 с.
- Бусев А. И., Симонова Л. Н. Аналитическая химия серы. - М.: Наука, 1975. - 272 с.
- Реми Г. Курс неорганической химии, т.1. М.: Издательство иностранной литературы, 1963. - 921 с.
- Гринвуд Н. Химия элементов: в 2 томах. М.: БИНОМ, 2008. 2 т. - 670 с.