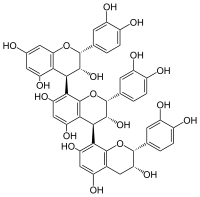
Procyanidin C1
 | |
| Names | |
|---|---|
| IUPAC name
(2R,2ʼR,2ʼʼR,3R,3ʼR,3ʼʼR,4R,4ʼS)-2,2ʼ,2ʼʼ-tris(3,4-dihydroxyphenyl)-3,3ʼ,3ʼʼ,4,4ʼ,4ʼʼ-hexahydro-2H,2ʼH,2ʼʼH-4,8ʼ:4ʼ,8ʼʼ-terchromene-3,3ʼ,3ʼʼ,5,5ʼ,5ʼʼ,7,7ʼ,7ʼʼ-nonol | |
| Other names
Procyanidin C1 Procyanidol C1 Epicatechin-(4.beta.-->8)epicatechin-(4.beta.-->8)epicatechin Epicatechin-(4β→8)-epicatechin--(4β→8)-epicatechin | |
| Identifiers | |
| 37064-30-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:75643 |
| ChEMBL | ChEMBL290632 |
| ChemSpider | 148540 |
| KEGG | C17624 |
| PubChem | 169853 |
| |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.77 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Procyanidin C1 is a B type proanthocyanidin. It is an epicatechin trimer found in grape (Vitis vinifera).[1]
Chemical synthesis
The stereoselective synthesis of seven benzylated proanthocyanidin trimers (epicatechin-(4β-8)-epicatechin-(4β-8)-epicatechin trimer (procyanidin C1), catechin-(4α-8)-catechin-(4α-8)-catechin trimer (procyanidin C2), epicatechin-(4β-8)-epicatechin-(4β-8)-catechin trimer and epicatechin-(4β-8)-catechin-(4α-8)-epicatechin trimer derivatives) can be achieved with TMSOTf-catalyzed condensation reaction, in excellent yields. The structure of benzylated procyanidin C2 was confirmed by comparing the 1H NMR spectra of protected procyanidin C2 that was synthesized by two different condensation approaches. Finally, deprotection of (+)-catechin and (-)-epicatechin trimers derivatives gives four natural procyanidin trimers in good yields.[2]
See also
References
- ↑ Proanthocyanidin composition of red Vitis vinifera varieties from the Douro valley during ripening : Influence of cultivation altitude. Mateus Nuno, Marques Sara, Goncalves Ana C., Machado José M. and De Freitas Victor, American journal of enology and viticulture, 2001, vol. 52, no2, pp. 115-121, INIST:1129642
- ↑ Efficient Stereoselective Synthesis of Proanthocyanidin Trimers with TMSOTf-Catalyzed Intermolecular Condensation. Akiko Saito, Akira Tanaka, Makoto Ubukata and Noriyuki Nakajima, Synlett, 2004, volume 6, pages 1069-1073, doi:10.1055/s-2004-822905