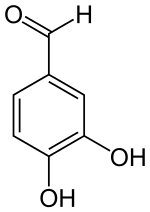
Protocatechuic aldehyde
 | |
| Names | |
|---|---|
| IUPAC name
3,4-dihydroxybenzaldehyde | |
| Other names
Protocatechualdehyde 3,4-Dihydroxybenzaldehyde Rancinamycin IV 3,4-Dihydroxybenzyl aldehyde | |
| Identifiers | |
| 139-85-5 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL222021 |
| ChemSpider | 8438 |
| ECHA InfoCard | 100.004.889 |
| PubChem | 8768 |
| |
| |
| Properties | |
| C7H6O3 | |
| Molar mass | 138.12 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Protocatechuic aldehyde is a phenolic aldehyde, a compound released from cork stoppers into wine.[1]
This molecule can be used as a precursor in the vanillin synthesis by biotransformation by cell cultures of Capsicum frutescens, a type of Chili pepper.[2] It is also found in the mushroom Phellinus linteus.[3]
References
- ↑ Polyphenolic Composition of Quercus suber Cork from Different Spanish Provenances. Elvira Conde, Estrella Cadahía, María Concepción García-Vallejo and Brígida Fernández de Simón, J. Agric. Food Chem., 1998, 46 (8), pp 3166–3171 doi:10.1021/jf970863k
- ↑ Biotransformation of protocatechuic aldehyde and caffeic acid to vanillin and capsaicin in freely suspended and immobilized cell cultures of Capsicum frutescens. Sathuluri Ramachandra Rao and Gokare Aswathanarayana Ravishankar, Journal of Biotechnology, Volume 76, Issues 2-3, 21 January 2000, Pages 137-146 doi:10.1016/S0168-1656(99)00177-7
- ↑ Lee YS, Kang YH, Jung JY, et al. (October 2008). "Protein glycation inhibitors from the fruiting body of Phellinus linteus". Biological & Pharmaceutical Bulletin. 31 (10): 1968–72. doi:10.1248/bpb.31.1968. PMID 18827365.
See also
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.