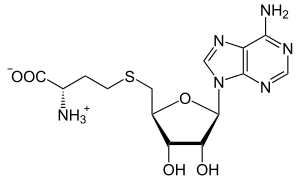
S-Adenosyl-L-homocysteine
 | |
| Names | |
|---|---|
| IUPAC name
S-(5'-Deoxyadenos-5'-yl)-L-homocysteine | |
| Other names
AdoHcy, 2-S-adenosyl-L-homocysteine, 5'-S-(3-Amino-3-carboxypropyl)-5'-thioadenosine S-adenosylhomocysteine, SAH | |
| Identifiers | |
| 979-92-0 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:16680 |
| ChEMBL | ChEMBL418052 |
| ChemSpider | 388301 |
| ECHA InfoCard | 100.012.328 |
| 5265 | |
| KEGG | C00021 |
| MeSH | S-Adenosylhomocysteine |
| PubChem | 439155 |
| |
| |
| Properties | |
| C14H20N6O5S | |
| Molar mass | 384.412 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
S-Adenosyl-L-homocysteine (SAH) is an amino acid derivative used in several metabolic pathways in most organisms. It is an intermediate in the synthesis of cysteine and adenosine.
SAH is formed by the demethylation of S-adenosyl-L-methionine (SAM).
External links
This article is issued from Wikipedia - version of the 2/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.