Sodium metaborate
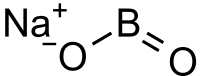 | |
| Identifiers | |
|---|---|
| 98536-58-4 | |
| 3D model (Jmol) | Interactive image |
| EC Number | 231-891-6 |
| PubChem | 145326 |
| RTECS number | ED4640000 |
| |
| |
| Properties | |
| NaBO2 | |
| Molar mass | 65.80 g/mol |
| Appearance | colorless crystals |
| Odor | odorless |
| Density | 2.46 g/cm3 |
| Melting point | 966 °C (1,771 °F; 1,239 K) |
| Boiling point | 1,434 °C (2,613 °F; 1,707 K) |
| 16.4 g/100 mL (0 °C) 28.2 g/100 mL (25 °C) 125.2 g/100 mL (100 °C) | |
| Solubility | insoluble in ether, ethanol |
| Structure | |
| trigonal | |
| Thermochemistry | |
| 65.94 J/mol K | |
| Std molar entropy (S |
73.39 J/mol K |
| Std enthalpy of formation (ΔfH |
-1059 kJ/mol |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) |
2330 mg/kg (rat, oral) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Sodium metaborate (NaBO2) is a colorless solid chemical compound. [1]
Preparation
Sodium metaborate is prepared by the fusion of sodium carbonate and borax. Another way to create the compound is by the fusion of sodium tetraborate with sodium hydroxide at 700° C.
Uses
Sodium metaborate is used in the manufacturing of borosilicate glasses. It is also a component of herbicides and antifreeze.
See also
References
This article is issued from Wikipedia - version of the 11/2/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.