Stephen aldehyde synthesis
Not to be confused with Stevens rearrangement.
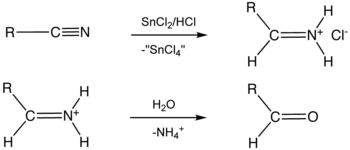
Stephen aldehyde synthesis, a named reaction in chemistry, was invented by Henry Stephen (OBE/MBE). This reaction involves the preparation of aldehydes (R-CHO) from nitriles (R-CN) using tin(II) chloride (SnCl2), hydrochloric acid (HCl) and quenching the resulting iminium salt ([R-CH=NH2]+Cl−) with water (H2O).[1][2] During the synthesis, ammonium chloride is also produced.
Overall, the reaction scheme is as follows:
Sonn-Müller method
In the Sonn-Müller method[3][4] the intermediate iminium salt is obtained from reaction of an amide PhCONHPh with phosphorus pentachloride.
See also
- Pinner reaction - a similar reaction using alcohols or amines as the nucleophile and without the reduction; generated esters, carboximidates or orthoesters.
- Nitrile reduction
- Amide reduction
References
- ↑ Williams, Jonathan W. (1943). "β-Naphthaldehyde". Organic Syntheses. 23: 63. doi:10.15227/orgsyn.023.0063.
- ↑ Stephen, H. (1925). "A new synthesis of aldehydes". J. Chem. Soc., Trans. 127: 1874–1877. doi:10.1039/CT9252701874.
- ↑ Adolf, Sonn; Müller, Ernst (1919). "Über eine neue Methode zur Umwandlung von Carbonsäuren in Aldehyde". Berichte der deutschen chemischen Gesellschaft (A and B Series). 52 (10): 1927–1934. doi:10.1002/cber.19190521002.
- ↑ Williams, Jonathan W.; Witten, Charles H.; Krynitsky, John A. (1946). "o-Tolualdehyde". Organic Syntheses. 26: 97. doi:10.15227/orgsyn.026.0097.
This article is issued from Wikipedia - version of the 7/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.