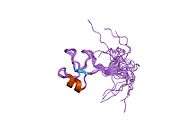
Trefoil factor 1
Trefoil factor 1 is a protein that in humans is encoded by the TFF1 gene[3][4] (also called pS2 gene[5]).
Function
Members of the trefoil family are characterized by having at least one copy of the trefoil motif, a 40-amino acid domain that contains three conserved disulfides. They are stable secretory proteins expressed in gastrointestinal mucosa. Their functions are not defined, but they may protect the mucosa from insults, stabilize the mucus layer, and affect healing of the epithelium. This gene, which is expressed in the gastric mucosa, has also been studied because of its expression in human tumors. This gene and two other related trefoil family member genes are found in a cluster on chromosome 21.[4]
In gastric carcinoma
TFF1 expression is frequently lost in gastric carcinoma, probably through mechanism of DNA methylation, and it is therefore considered as a tumor suppressor gene.[6]
References
- ↑ "Human PubMed Reference:".
- ↑ "Mouse PubMed Reference:".
- ↑ Gött P, Beck S, Machado JC, Carneiro F, Schmitt H, Blin N (May 1997). "Human trefoil peptides: genomic structure in 21q22.3 and coordinated expression". Eur J Hum Genet. 4 (6): 308–15. PMID 9043862.
- 1 2 "Entrez Gene: TFF1 trefoil factor 1".
- ↑ Chatagnon A, Ballestar E, Esteller M, Dante R (2010). "A Role for Methyl-CpG Binding Domain Protein 2 in the Modulation of the Estrogen Response of pS2/TFF1 Gene". PLoS ONE. 5 (3): e9665. doi:10.1371/journal.pone.0009665. PMC 2837351
 . PMID 20300195.
. PMID 20300195. - ↑ Feng G, Zhang Y, Yuan H, Bai R, Zheng J, Zhang J, Song M (Jan 2014). "DNA methylation of trefoil factor 1 (TFF1) is associated with the tumorigenesis of gastric carcinoma.". J Mol Med Rep. 9 (1): 109–117. doi:10.3892/mmr.2013.1772. PMID 24190027.
Further reading
- Langer G, Jagla W, Behrens-Baumann W, Walter S, Hoffmann W (2003). "Ocular TFF-peptides: new mucus-associated secretory products of conjunctival goblet cells.". Adv. Exp. Med. Biol. 506 (Pt A): 313–6. PMID 12613926.
- Piggott NH, Henry JA, May FE, Westley BR (1991). "Antipeptide antibodies against the pNR-2 oestrogen-regulated protein of human breast cancer cells and detection of pNR-2 expression in normal tissues by immunohistochemistry.". J. Pathol. 163 (2): 95–104. doi:10.1002/path.1711630204. PMID 1707960.
- Mori K, Fujii R, Kida N, Takahashi H, Ohkubo S, Fujino M, Ohta M, Hayashi K (1990). "Complete primary structure of the human estrogen-responsive gene (pS2) product.". J. Biochem. 107 (1): 73–6. PMID 2185238.
- Tomasetto C, Rio MC, Gautier C, Wolf C, Hareuveni M, Chambon P, Lathe R (1990). "hSP, the domain-duplicated homolog of pS2 protein, is co-expressed with pS2 in stomach but not in breast carcinoma.". EMBO J. 9 (2): 407–14. PMC 551681
 . PMID 2303034.
. PMID 2303034.
- Takahashi H, Kida N, Fujii R, Tanaka K, Ohta M, Mori K, Hayashi K (1990). "Expression of the pS2 gene in human gastric cancer cells derived from poorly differentiated adenocarcinoma.". FEBS Lett. 261 (2): 283–6. doi:10.1016/0014-5793(90)80572-Z. PMID 2311759.
- Rio MC, Bellocq JP, Daniel JY, Tomasetto C, Lathe R, Chenard MP, Batzenschlager A, Chambon P (1988). "Breast cancer-associated pS2 protein: synthesis and secretion by normal stomach mucosa.". Science. 241 (4866): 705–8. doi:10.1126/science.3041593. PMID 3041593.
- Rio MC, Lepage P, Diemunsch P, Roitsch C, Chambon P (1989). "[Primary structure of human protein pS2]". C. R. Acad. Sci. III, Sci. Vie. 307 (19): 825–31. PMID 3146413.
- Mori K, Fujii R, Kida N, Ohta M, Hayashi K (1988). "Identification of a polypeptide secreted by human breast cancer cells (MCF-7) as the human estrogen-responsive gene (pS2) product.". Biochem. Biophys. Res. Commun. 155 (1): 366–72. doi:10.1016/S0006-291X(88)81094-5. PMID 3261981.
- Jeltsch JM, Roberts M, Schatz C, Garnier JM, Brown AM, Chambon P (1987). "Structure of the human oestrogen-responsive gene pS2.". Nucleic Acids Res. 15 (4): 1401–14. doi:10.1093/nar/15.4.1401. PMC 340557
 . PMID 3822834.
. PMID 3822834.
- Prud'homme JF, Fridlansky F, Le Cunff M, Atger M, Mercier-Bodart C, Pichon MF, Milgrom E (1985). "Cloning of a gene expressed in human breast cancer and regulated by estrogen in MCF-7 cells.". DNA. 4 (1): 11–21. doi:10.1089/dna.1985.4.11. PMID 3838275.
- Jakowlew SB, Breathnach R, Jeltsch JM, Masiakowski P, Chambon P (1984). "Sequence of the pS2 mRNA induced by estrogen in the human breast cancer cell line MCF-7.". Nucleic Acids Res. 12 (6): 2861–78. doi:10.1093/nar/12.6.2861. PMC 318711
 . PMID 6324130.
. PMID 6324130.
- Hanby AM, Poulsom R, Singh S, Elia G, Jeffery RE, Wright NA (1993). "Spasmolytic polypeptide is a major antral peptide: distribution of the trefoil peptides human spasmolytic polypeptide and pS2 in the stomach.". Gastroenterology. 105 (4): 1110–6. PMID 8405856.
- Polshakov VI, Frenkiel TA, Westley B, Chadwick M, May F, Carr MD, Feeney J (1996). "NMR-based structural studies of the pNR-2/pS2 single domain trefoil peptide. Similarities to porcine spasmolytic peptide and evidence for a monomeric structure.". Eur. J. Biochem. 233 (3): 847–55. doi:10.1111/j.1432-1033.1995.847_3.x. PMID 8521850.
- Seib T, Blin N, Hilgert K, Seifert M, Theisinger B, Engel M, Dooley S, Zang KD, Welter C (1997). "The three human trefoil genes TFF1, TFF2, and TFF3 are located within a region of 55 kb on chromosome 21q22.3.". Genomics. 40 (1): 200–2. doi:10.1006/geno.1996.4511. PMID 9070946.
- Polshakov VI, Williams MA, Gargaro AR, Frenkiel TA, Westley BR, Chadwick MP, May FE, Feeney J (1997). "High-resolution solution structure of human pNR-2/pS2: a single trefoil motif protein.". J. Mol. Biol. 267 (2): 418–32. doi:10.1006/jmbi.1997.0896. PMID 9096235.
- Chadwick MP, Westley BR, May FE (1997). "Homodimerization and hetero-oligomerization of the single-domain trefoil protein pNR-2/pS2 through cysteine 58.". Biochem. J. 327 (1): 117–23. PMC 1218770
 . PMID 9355742.
. PMID 9355742.
- Chen H, Lin RJ, Xie W, Wilpitz D, Evans RM (1999). "Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of an acetylase.". Cell. 98 (5): 675–86. doi:10.1016/S0092-8674(00)80054-9. PMID 10490106.
- Newton JL, Allen A, Westley BR, May FE (2000). "The human trefoil peptide, TFF1, is present in different molecular forms that are intimately associated with mucus in normal stomach.". Gut. 46 (3): 312–20. doi:10.1136/gut.46.3.312. PMC 1727855
 . PMID 10673290.
. PMID 10673290.
PDB gallery |
|---|
|
| 1hi7: NMR SOLUTION STRUCTURE OF THE DISULPHIDE-LINKED DIMERIC OF HUMAN TFF1, 10 STRUCTURES |
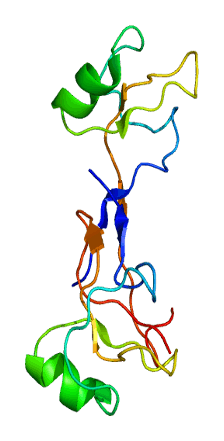
| 1ps2: HIGH RESOLUTION NMR SOLUTION STRUCTURE OF HUMAN PS2, 19 STRUCTURES |
|
|

 . PMID 20300195.
. PMID 20300195. . PMID 2303034.
. PMID 2303034. . PMID 3822834.
. PMID 3822834. . PMID 6324130.
. PMID 6324130. . PMID 9355742.
. PMID 9355742. . PMID 10673290.
. PMID 10673290.