Tagetitoxin
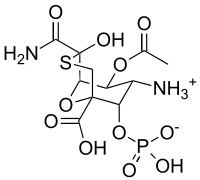 Proposed structure of tagetitoxin | |
| Identifiers | |
|---|---|
| 87913-21-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 103185 |
| PubChem | 115340 |
| |
| |
| Properties | |
| C11H17N2O11PS | |
| Molar mass | 416.29 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tagetitoxin (TGT) is a bacterial phytotoxin produced by Pseudomonas syringae pv. tagetis.[1][2]
Chemical structure
When TGT was first isolated, it was only partially characterized.[2] The first proposed chemical structure of TGT involved an eight-membered ring,[3] but this was revised shortly afterward to a bicyclic structure (shown at right) based on NMR and mass spectrometry.[4] This structure, however, has been questioned.[5] The absolute configuration remains undetermined, and attempts at confirming the structure by organic synthesis are underway.[6][7][8][9][10][11][12]
Mechanism of action
TGT interferes with development of chloroplasts in young plant leaves thereby causing chlorosis.[13] The natural target of the toxin is chloroplast RNA polymerase. Chloroplast RNA polymerase belongs to ubiquitous family of multisubunit RNA polymerases (RNAP) and is most closely related to bacterial enzymes. In vitro, TGT inhibits bacterial RNAPs from Escherichia coli and Thermus thermophilus, and eukaryotic RNA polymerase III.[14] In contrast, eukaryotic RNA polymerase I and II as well as single-subunit RNA polymerases of bacteriophage T7 and SP6 are relatively insensitive to the compound. TGT binds in the RNAP active site[15] and inhibits initiation and elongation phases of transcription as well as pyrophosphorolysis of the nascent RNA.[15] However, the detailed mechanism of inhibition remains a subject of heated debate.[16][17]
It has been suggested that TGT forms a ternary RNAP-NTP-TGT complex and inhibits phosphodiester bond synthesis either by binding an inhibitory magnesium ion[15] or by trapping a flexible active site domain in an inactive conformation.[18] The third theory suggests that TGT forms predominantly a binary RNAP-TGT complex and inhibits RNAP translocation along the DNA by mimicking the transcription byproduct pyrophosphate.[19]
References
- ↑ Trimboli, D; Fahy, PC; Baker, KF (1978). "Apical chlorosis and leaf spot of Tagetes spp. Caused by Pseudomonas tagetis Hellmers". Australian Journal of Agricultural Research. 29 (4): 831–9. doi:10.1071/AR9780831.
- 1 2 Mitchell, R. E.; Durbin, R. D. (1981). "Tagetitoxin, a toxin produced by Pseudomonas syringae pv. tagetis: purification and partial characterization". Physiological Plant Pathology. 18 (2): 157–68. doi:10.1016/S0048-4059(81)80037-9.
- ↑ Mitchell, R. E.; Durbin, R. D. (1983). "The structure of tagetitoxin, a phytotoxin of Pseudomonas syringae pv. Tagetis". Phytochemistry. 22 (6): 1425–1428. doi:10.1016/S0031-9422(00)84028-5.
- ↑ Mitchell, R. E.; Coddington, J. M.; Young, H. (1989). "A revised structure for tagetitoxin". Tetrahedron Lett. 30 (4): 501–504. doi:10.1016/S0040-4039(00)95239-0.
- ↑ Gronwald, J.W.; Plaisance, K. L.; Marimanikkuppam, S.; Ostrowski, B. G. (2005). "Tagetitoxin purification and partial characterization". Physiol. Mol. Plant Pathol. 67: 23–32. doi:10.1016/j.pmpp.2005.09.002.
- ↑ Porter, Michael; Plet, Julien; Sandhu, Amandeep; Sehailia, Moussa (2009). "Thieme Chemistry Journal Awardees - Where Are They Now? Approaches to Tagetitoxin and its Decarboxy Analogue from d-Glucose". Synlett. 2009 (20): 3258–3262. doi:10.1055/s-0029-1218525.
- ↑ Mortimer, Anne J. Price; Aliev, Abil E.; Tocher, Derek A.; Porter, Michael J. (2008). "Synthesis of the Tagetitoxin Core via Photo-Stevens Rearrangement". Organic Letters. 10 (23): 5477–80. doi:10.1021/ol802297h. PMID 18973329.
- ↑ Sammakia, T.; Hurley, T. B.; Sammond, D. M.; Smith, R. S.; Sobolov, S. B.; Oeschger, T. R. (1996). "Dihydroxylation and oxidative cleavage of olefins in the presence of sulfur". Tetrahedron Lett. 37 (26): 4427–4430. doi:10.1016/0040-4039(96)00879-9.
- ↑ Dent, B. R.; Furneaux, R. H.; Gainsford, G. J.; Lynch, G. P. (1999). "Synthesis studies of structural analogues of tagetitoxin: 2-phosphate". Tetrahedron. 55 (22): 6977–6996. doi:10.1016/S0040-4020(99)00327-0.
- ↑ Plet, Julien R. H.; Porter, Michael J. (2006). "Synthesis of the bicyclic core of tagetitoxin". Chemical Communications (11): 1197–9. doi:10.1039/B600819D. PMID 16518489.
- ↑ Mortimer, Anne J. P.; Plet, Julien R. H.; Obasanjo, Oluwafunsho A.; Kaltsoyannis, Nikolas; Porter, Michael J. (2012). "Inter- and intramolecular reactions of 1-deoxy-1-thio-1,6-anhydrosugars with α-diazoesters: synthesis of the tagetitoxin core by photochemical ylide rearrangement". Org. Biomol. Chem. 10 (43): in press. doi:10.1039/c2ob26308d.,
- ↑ Sehailia, Moussa (2011). Studies towards the total synthesis of tagetitoxin (Doctoral thesis). University College London.
- ↑ Lukens, J. H.; Durbin, R. D. (1985). "Tagetitoxin affects plastid development in seedling leaves of wheat". Planta. 165 (3): 311–21. doi:10.1007/BF00392227. PMID 24241135.
- ↑ Steinberg, Thomas H.; Mathews, Dennis E.; Durbin, Richard D.; Burgess, Richard R. (1990). "Tagetitoxin: A New Inhibitor of Eukaryotic Transcription by RNA Polymerase III". The Journal of Biological Chemistry. 265 (1): 499–505. PMID 2403565.
- 1 2 3 Vassylyev, Dmitry G; Svetlov, Vladimir; Vassylyeva, Marina N; Perederina, Anna; Igarashi, Noriyuki; Matsugaki, Naohiro; Wakatsuki, Soichi; Artsimovitch, Irina (2005). "Structural basis for transcription inhibition by tagetitoxin". Nature Structural & Molecular Biology. 12 (12): 1086–93. doi:10.1038/nsmb1015. PMC 1790907
 . PMID 16273103.
. PMID 16273103. - ↑ Klyuyev, Sergiy; Vassylyev, Dmitry G. (2012). "The binding site and mechanism of the RNA polymerase inhibitor tagetitoxin: An issue open to debate". Transcription. 3 (2): 46–50. doi:10.4161/trns.19468. PMC 3337825
 . PMID 22414754.
. PMID 22414754. - ↑ Svetlov, Vladimir; Artsimovitch, Irina; Nudler, Evgeny (2012). "Response to Klyuyev and Vassylyev: On the mechanism of tagetitoxin inhibition of transcription". Transcription. 3 (2): 51–5. doi:10.4161/trns.19749. PMC 3337826
 . PMID 22414748.
. PMID 22414748. - ↑ Artsimovitch, Irina; Svetlov, Vladimir; Nemetski, Sondra Maureen; Epshtein, Vitaly; Cardozo, Timothy; Nudler, Evgeny (2011). "Tagetitoxin Inhibits RNA Polymerase through Trapping of the Trigger Loop". The Journal of Biological Chemistry. 286 (46): 40395–400. doi:10.1074/jbc.M111.300889. PMC 3220573
 . PMID 21976682.
. PMID 21976682. - ↑ Malinen, Anssi M.; Turtola, Matti; Parthiban, Marimuthu; Vainonen, Lioudmila; Johnson, Mark S.; Belogurov, Georgiy A. (2012). "Active site opening and closure control translocation of multisubunit RNA polymerase". Nucleic Acids Research. 40 (15): 7442–51. doi:10.1093/nar/gks383. PMC 3424550
 . PMID 22570421.
. PMID 22570421.