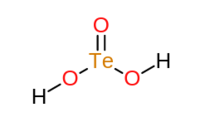
Tellurous acid
 | |
| Names | |
|---|---|
| IUPAC name
Tellurous acid | |
| Other names
Tellurium dioxide hydrate, tellurium(IV) oxide hydrate | |
| Identifiers | |
| 10049-23-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:30465 |
| ChemSpider | 23310 |
| ECHA InfoCard | 100.030.145 |
| PubChem | 24936 |
| UNII | IVA6SGP6QM |
| |
| |
| Properties | |
| H2TeO3 | |
| Molar mass | 177.616 grams |
| Appearance | colorless crystals |
| Density | ~ 3 g/cm3 |
| Boiling point | decomposes |
| negligible | |
| Acidity (pKa) | pKa(1) = 2.48, pKa(2) = 7.70 [1] |
| Structure | |
| unknown | |
| pyramidal at Te | |
| Related compounds | |
| Other anions |
Selenous acid Sulfurous acid |
| Other cations |
Sodium tellurite |
| Related compounds |
Telluric acid Selenic acid Sulfuric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tellurous acid is an inorganic compound with the formula H2TeO3. It is the oxoacid of tellurium(IV).[2] The compound is not well characterized. An alternative way of writing its formula is (HO)2TeO. In principle, tellurous acid would form by treatment of tellurium dioxide with water, i.e. hydrolysis. The related conjugate base is well known in the form of several salts such as potassium acid tellurite, KHTeO3.
Properties
In contrast to the analogous compound Selenous acid, Tellurous acid is only metastable.
Most tellurite salts contain the TeO2−
3 ion. Oxidation of its aqueous solution with hydrogen peroxide gives the tellurate ion.
It is usually prepared as an aqueous solution where it acts as a weak acid.
[1]
- H2TeO3 + H2O ⇄ H3O+ + HTeO−
3 Ka1 = 2 x 10−3
- HTeO−
3 + H2O ⇄ H3O+ + TeO2−
3 Ka2 = 1 x 10−8[3]
References
- 1 2 Catherine E. Housecroft; Alan G. Sharpe (2008). "Chapter 16: The group 16 elements". Inorganic Chemistry, 3rd Edition. Pearson. p. 524. ISBN 978-0-13-175553-6.
- ↑ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ http://www.austincc.edu/chemlab/weakacid
This article is issued from Wikipedia - version of the 10/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.