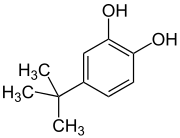
4-tert-Butylcatechol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4-tert-Butylbenzene-1,2-diol | |
| Other names
para-tert-Butylcatechol p-tert-Butylcatechol t-Butyl catechol p-t-Butylpyrocatechol p-tert-Butylpyrocatechol 4-t-Butylpyrocatechol 4-tert-Butylpyrocatechol | |
| Identifiers | |
| 98-29-3 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | TBC |
| ChEMBL | ChEMBL220845 |
| ChemSpider | 7103 |
| ECHA InfoCard | 100.002.413 |
| PubChem | 7381 |
| |
| |
| Properties | |
| C10H14O2 | |
| Molar mass | 166.217 g/mol |
| Melting point | 50 °C |
| Boiling point | 285 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
4-tert-Butylcatechol (TBC) is an organic chemical compound which is a derivative of catechol. It is added as a stabilizer and an inhibitor of polymerization to butadiene, styrene, vinyl acetate and other reactive monomer streams. It is 25 times better than hydroquinone at 60 °C for polymerization inhibitory effect. Also used as a stabilizer in the manufacture of polyurethane foam. It also can be used as an antioxidant for synthetic rubber, polymers and oil derivatives. It can be used as purification agent for aminoformate catalysts. TBC is available in a form of a solid crystal and 85% solution in methanol or water.
See also
- tert-Butylhydroquinone
- US Patent 4061545:Polymerization inhibitor for vinyl aromatic compounds
- US Patent 3046472: Substituted Catechol Antioxidants
References
This article is issued from Wikipedia - version of the 9/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.