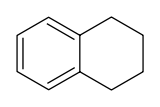
Tetralin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,2,3,4-tetrahydronaphthalene | |
| Other names
naphthalene 1,2,3,4-tetrahydride Bacticin benzocyclohexane THN | |
| Identifiers | |
| 119-64-2 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:35008 |
| ChemSpider | 8097 |
| ECHA InfoCard | 100.003.946 |
| KEGG | C14114 |
| PubChem | 8404 |
| UNII | FT6XMI58YQ |
| |
| |
| Properties | |
| C10H12 | |
| Molar mass | 132.21 g·mol−1 |
| Appearance | Clear, colorless liquid with an odor similar to naphthalene |
| Density | 0.970 g/cm3 |
| Melting point | −35.8 °C (−32.4 °F; 237.3 K) |
| Boiling point | 206 to 208 °C (403 to 406 °F; 479 to 481 K) |
| Insoluble | |
| Viscosity | 2.02 cP at 25 °C[1] |
| Hazards | |
| Safety data sheet | JT Baker MSDS |
| Flash point | 77 °C (171 °F; 350 K) |
| 385 °C (725 °F; 658 K) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetralin (1,2,3,4-tetrahydronaphthalene) is a hydrocarbon having the chemical formula C10H12. This molecule is similar to the naphthalene chemical structure except that one ring is saturated.
Synthesis
The compound can be synthesized in a Bergman cyclization whereby cyclodeca-3-ene-1,5-diyne reacts with 1,3-cyclohexadiene to produce benzene and tetralin. In a classic named reaction called the Darzens tetralin synthesis, named for Auguste Georges Darzens (1926), derivatives can be prepared by intramolecular electrophilic aromatic substitution reaction of an 1-aryl-4-pentene using concentrated sulfuric acid,[2]
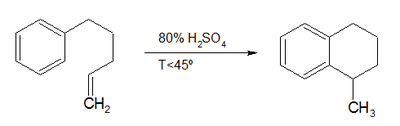
It can also be prepared by partial hydrogenation of naphthalene in the presence of a platinum catalyst.
Production
Tetralin is produced by the catalytic hydrogenation of naphthalene.
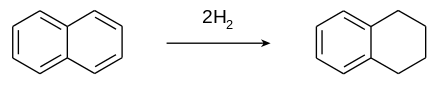
A large amount of work has been devoted to the hydrogenation of naphthalene into tetralin. Mixtures containing different proportions of naphthalene, tetralin, and decalin can be produced depending on the pressure and temperature of the process. Tetralin has been obtained from pressed naphthalene isolated from coal tar by hydrogenating it over commercial catalyst WS2 + NiS + Al2O3 and CoO + MoO3 + Al2O3 under pressure of 50–300 atm. The extent to which naphthalene is hydrogenated at pressures up to 60–70 atm is determined by catalyst activity; it is maximum if CoO + MoO3 + Al2O3 or WS2 + NiS + Al2O3 catalyst are used at 300–370 °C.[3]
Uses
Tetralin is used as a solvent.
It is also used for the laboratory synthesis of dry HBr gas:
- C10H12 + 4 Br2 → C10H8Br4 + 4 HBr
See also
References
- ↑ Gonçalves, F. A.; Hamano, K.; Sengers, J. V. (1989). "Density and viscosity of tetralin and trans-decalin". International Journal of Thermophysics. 10 (4): 845. doi:10.1007/BF00514480.
- ↑ Michael B. Smith (2011). Organic Synthesis (third ed.). Academic Press. pp. 1209–1210. ISBN 9780124158849.
- ↑ Krichko, A. A.; Skvortsov, D. V.; Titova, T. A.; Filippov, B. S.; Dogadkina, N. E. (1969). "Production of tetralin by the hydrogenation of naphthalene-containing fractions". Chemistry and Technology of Fuels and Oils. 5: 18. doi:10.1007/BF00727949.