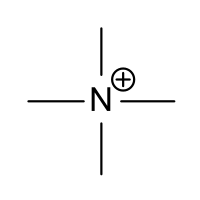
Tetramethylammonium
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
N,N,N-Trimethylmethanaminium[1] | |
| Other names | |
| Identifiers | |
| 51-92-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:46020 |
| ChemSpider | 6140 |
| PubChem | 6380 |
| |
| |
| Properties | |
| C4H12N+ | |
| Molar mass | 74.14 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetramethylammonium (TMA) or (Me4N+) is the simplest quaternary ammonium cation consisting of four methyl groups attached to a central nitrogen atom, and is isoelectronic with neopentane. It is positively charged and must exist in association with a counter-ion, and is most commonly found in simple salts such as tetramethylammonium chloride, tetramethylammonium bromide, tetramethylammonium iodide and tetramethylammonium hydroxide, and can also form the unusual auride. Tetramethylammonium salts are used in chemical synthesis and are widely employed in pharmacological research. They also occur as toxic natural products, although the counterions may be difficult to identify. The identity of the anion associated with the tetramethylammonium cation frequently has little or no bearing on a particular chemical or biological type of action, but this is not invariably so.
Common nomenclature
In the toxicological literature, naturally-occurring tetramethylammonium (anion unspecified) is often referred to by the name "tetramine". Unfortunately, this non-systematic or "trivial" name is also used for other chemical entities, including a toxic rodenticide (Tetramethylenedisulfotetramine). Similarly, the acronym "TMA", which is frequently used for tetramethylammonium in the pharmacological literature, may also refer to the investigational drug 3,4,5-trimethoxyamphetamine, which, being a close structural analog of mescaline, has been the subject of numerous publications.
For the sake of convenience, and consistency with the Wikipedia entry for tetraethylammonium, the abbreviation "TMA" will be used in this article.
Occurrence
TMA has been detected in or isolated from a number of marine organisms, mostly amongst the Cnidaria and Mollusca, notably in some species of Neptunea (commonly called whelks) that are eaten by humans.[2][3] It has also been found in one plant, the African Courbonia virgata (Cappariaceae).[4]
Chemistry
Preparation
One of the most straightforward methods of preparing a simple salt containing the tetramethylammonium ion is by the reaction between trimethylamine and a methyl halide, e.g.
Me3N + Me-I → Me4N+I−
[14C]-labeled TMA has been made by this method.[5]
Although this reaction is suitable for the common halides, tetramethylammonium salts with more complex anions may be prepared by salt metathesis reactions, e.g. tetramethylammonium borohydride has been made from tetramethylammonium hydroxide as shown:[6]
Me4N+[OH]− + Na+[BH4]− → Me4N+[BH4]− + Na+ + HO−
Synthetic applications
The synthetic applications of TMA are numerous and diverse, but are most appropriately considered under the entries for individual TMA salts (e.g. tetramethylammonium chloride and tetramethylammonium hydroxide). In general, although TMA salts do possess some of the phase-transfer catalytic properties that are characteristic of quaternary ammonium compounds, they tend to behave atypically because of the relatively high hydrophilicity of the TMA cation.[7]
Properties
In the TMA cation, the methyl groups are tetrahedrally arranged around the central N atom, as is evident from X-ray crystallographic studies of various of its salts.[8][9] From measurements taken on molecular models, it has been estimated that the diameter of the TMA ion is ~ 0.6 nm;[10] In more recent studies, based on more accurate physico-chemical measurements, the ionic radius for TMA is given as 0.322 nm; several thermodynamic parameters for the TMA ion are also recorded.[11][12] The paper by Aue et al. gives a good discussion of the methods by which the ionic radius was determined.[11]
A recent study of the TMA cation indicates that it has unexpectedly hydrophilic properties, despite the presence of four methyl groups in its structure.[13]
The partition coefficient of TMA iodide in octanol-water, Po-w was determined experimentally to be 0.12 x 10−3 (or log P ≅ -3.92).[5]
Biology
Pharmacology
The literature dealing with the pharmacologically-related properties of tetramethylammonium is extensive, and research continues.[14] In general, TMA is a cholinomimetic whose effects mimic most of those produced by exogenous acetylcholine.[15]
Pharmacological experiments with TMA have been performed using one of its salts, typically the chloride, bromide or iodide, since these anions were not expected to interfere with the actions of the TMA cation. In the early pharmacological literature, however, there are references to the use of "tetramethylammonium hydroxide" or "tetramethylammonium hydrate", which were meant to facilitate comparison between weight-based dosages of different TMA salts,[16] but did not involve the actual use of tetramethylammonium hydroxide, whose strong basicity would have been incompatible with physiological conditions.[2]
A thorough review of the pharmacology of TMA from a toxicological perspective, and current up to 1989, has been given by Anthoni and co-workers.[2] Thus, the effects of TMA on nicotinic and muscarinic ACh receptors first stimulate, then block neurotransmission in sympathetic and parasympathetic ganglia, with depolarization. TMA also acts as an agonist at muscarinic receptors in post-ganglionic nerve endings in smooth muscles, cardiac muscle, and exocrine glands. In skeletal muscle, TMA initially causes fasciculations, then paralysis, as a result of the depolarization from stimulation of nicotinic ACh receptors.
Absorption; distribution; metabolism; excretion (ADME)
Absorption: TMA is readily absorbed from the gastro-intestinal tract.[2] Studies on the rat jejunum indicated that TMA absorption involved a combination of simple diffusion and carrier-mediated transport, with nearly 100% absorption occurring within 60-90 mins. By comparison, tetraethylammonium and tetrapropylammonium ions were only absorbed to the extent of ~ 30%.[17]
Distribution: Intraperitoneal administration of radio-labeled tetramethylammonium iodide to mice showed that TMA was rapidly distributed to all parts of the body, with the highest concentrations being in the kidney and liver.[5] Similar results were reported by Neef and co-workers using rats.[18]
Metabolism/Excretion: Parenteral administration of radio-labeled tetramethylammonium iodide to rats resulted in almost the whole dose being excreted in urine, without any evidence of metabolic transformation.[18]
Toxicology
The human toxicology of TMA (under the name "tetramine" - see "Common Nomenclature", above) has been studied primarily in the context of accidental poisoning after ingestion of Neptunea species.[2] Symptoms include the following: nausea, vomiting, headache, vertigo/dizziness, impaired vision/temporary blindness, diplopia, photophobia, lack of balance, feeling of intoxication and urticaria. These symptoms appear within 30 minutes but recovery is usually complete after a few hours. Only one account of human death following ingestion of TMA (from the plant Courbonia virgata) has been recorded.[4] Although many of these symptoms can be accounted for on the basis of impairment of neurotransmission in the autonomic nervous system, there also seem to be distinct indications of central affects.[2]
In animal studies, parenteral administration of TMA-containing extracts from Neptunea to mice, cats and fish mainly show effects involving skeletal muscles: there are muscular fasciculations, convulsions, loss of balance, motor paralysis and ultimately cessation of respiration.[2]
The lethal oral dose of TMA for humans has been estimated at 3–4 mg/kg.[2][4] The lethal dose for rats was estimated to be ~ 45–50 mg/kg, p.o., and ~ 15 mg/kg, i.p.[19]
Acute toxicity
LD50 for TMA chloride: 25 mg/kg (mouse, i.p.); 40 mg/kg (mouse, s.c.). LC50 for TMA chloride: 462 mg/L for 96 hrs. (Fathead minnow, Pimephales promelas). [20][21]
See also
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 1086. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 6 7 8 Anthoni, U.; Bohlin, L.; Larsen, C.; Nielsen, P.; Nielsen, N. H.; Christophersen, C. (1989). "Tetramine: Occurrence in marine organisms and pharmacology". Toxicon. 27: 707–716. doi:10.1016/0041-0101(89)90037-8.
- ↑ Dolan, L. C.; Matulka, R. A.; Burdock, G. A. (2010). "Naturally occurring food toxins". Toxins (Basel). 2: 2289–2332. doi:10.3390/toxins2092289. PMC 3153292
 . PMID 22069686.
. PMID 22069686. - 1 2 3 Henry, A. J. (1948). "The toxic principle of Courbonia virgata: its isolation and identification as a tetramethylammonium salt". Br. J. Pharmacol. Chemother. 3: 187–188. doi:10.1111/j.1476-5381.1948.tb00373.x. PMC 1509833
 . PMID 18883998.
. PMID 18883998. - 1 2 3 Tsubaki, H.; Nakajima, E.; Komai, T.; Shindo, H. (1986). "The relation between structure and distribution of quaternary ammonium ions in mice and rats. Simple tetraalkylammonium and a series of m-substituted trimethylphenylammonium ions". J. Pharmacobio-Dyn. 9: 737–746. doi:10.1248/bpb1978.9.737.
- ↑ Banus, M. D.; Bragdon, R. W.; Gibb, T. R. P. (1952). "Preparation of quaternary ammonium borohydrides from sodium and lithium borohydrides". J. Am. Chem. Soc. 74: 2346–2348. doi:10.1021/ja01129a048.
- ↑ Fedorynski, M.; Ziolkowska, W.; Jonczyk, A. (1993). "Tetramethylammonium salts: highly selective catalysts for the preparation of gem-dichlorocyclopropanes from electrophilic alkenes and chloroform under phase-transfer catalysis conditions". J. Org. Chem. 58: 6120–6121. doi:10.1021/jo00074a047.
- ↑ McLean, W. J.; Jeffrey, G. A. (1967). "Crystal structure of tetramethylammonium fluoride tetrahydrate". J. Chem. Phys. 47: 414–417. doi:10.1063/1.1711910.
- ↑ McCullough, J. D. (1964). "The crystal structure of tetramethylammonium perchlorate". Acta Crystallogr. 17: 1067–1070. doi:10.1107/s0365110x64002687.
- ↑ McCleskey, E. W.; Almers, W. (1985). "The Ca channel in skeletal muscle is a large pore". Proc. Natl. Acad. Sci. USA. 82: 7149–7153. doi:10.1073/pnas.82.20.7149.
- 1 2 Aue, D. H.; Webb, H. M.; Bowers, M. T. (1976). "A thermodynamic analysis of solvation effects on the basicities of alkylamines. An electrostatic analysis of substitutent effects". J. Am. Chem. Soc. 98: 318–329. doi:10.1021/ja00418a002.
- ↑ Palomo, J.; Pintauro, P. N. (2003). "Competitive absorption of quaternary ammonium and alkali metal cations into a Nafion cation-exchange membrane". J. Membrane Sci. 215: 103–114. doi:10.1016/s0376-7388(02)00606-3.
- ↑ Koga, Y.; Westh, P.; Nishikawa, K.; Subramanian, S. (2011). "Is a methyl group always hydrophobic? Hydrophilicity of trimethylamine-N-oxide, tetramethyl urea and tetramethylammonium ion". J. Phys. Chem. B. 115: 2995–3002. doi:10.1021/jp108347b.
- ↑ There are over 1300 citations in PubMed, as of October 2012.
- ↑ Drill's Pharmacology in Medicine, 4th Ed. (1971), J. R. DiPalma, Ed., p. McGraw-Hill, NJ.
- ↑ Burn, J. H.; Dale, H. H. (1915). "The action of certain quaternary ammonium bases". J. Pharmacol. Exp. Ther. 6: 417–438.
- ↑ Tsubaki, H.; Komai, T. (1986). "Intestinal absorption of tetramethylammonium and its derivatives in rats". J. Pharmacobio-Dyn. 9: 747–754. doi:10.1248/bpb1978.9.747.
- 1 2 Neef, C.; Oosting, R.; Meijer, D. K. F (1984). "Structure-pharmacokinetics relationship of quaternary ammonium compounds". Naunyn-Schmiedebergs Arch. Pharmakol. 328: 103–110. doi:10.1007/bf00512058.
- ↑ Anthoni, U.; Bohlin, L.; Larsen, C.; Nielsen, P.; Nielsen, N. H.; Christophersen, C. (1989). "The toxin tetramine from the "edible" whelk Neptunea antiqua". Toxicon. 27: 717–723. doi:10.1016/0041-0101(89)90038-x.
- ↑ R. J. Lewis (Ed.) (2004), Sax's Dangerous Properties of Industrial Materials, 11th Ed. p. 3409, Wiley-Interscience, Wiley & Sons, Inc., Hoboken, NJ.
- ↑ http://toxnet.nlm.nih.gov/cgi-bin/sis/search/a?dbs+hsdb:@term+@DOCNO+7987