Thermodynamics of micellization
The surfactant’s critical micelle concentration (CMC) plays a factor in Gibbs free energy of micellization. The exact concentration of the surfactants that yield the aggregates being thermodynamically soluble is the CMC. The Krafft temperature determines the solubility of the surfactants which in turn is the temperature that CMC is achieved. There are many parameters that affect the CMC. The interaction between the hydrophilic heads and the hydrophobic tails play a part, as well as the concentration of salt within the solution and surfactants.
Micelle
A micelle is an aggregation of surfactants in aqueous solution, often spherical.
Surfactants
Surfactants are composed of a polar head group that is hydrophilic and a nonpolar tail group that is hydrophobic. The head groups can be anionic, cationic, zwitterionic, or nonionic. The tail group can be a hydrocarbon, fluorocarbon, or a siloxane. Extensive variation in the surfactant’s solution and interfacial properties is allowed through different molecular structures of surfactants.[1]
Hydrophobic coagulation occurs when a positively charged solution is added with a sodium alkyl sulfate. The coagulation value is smaller when the alkyl chain length of the coagulator is longer. Hydrophobic coagulation occurs when a negatively charged solution contains a cationic surfactant. The coulomb attraction between the head groups and surface competes with the hydrophobic attraction for the entire tail in a favorable manner.[1]
Gibbs free energy of micellization
In general, the Gibbs free energy of micellization can be approximated as:
where is the molar Gibbs energy of micellization, is the universal gas constant, is the absolute temperature, and is the critical micelle concentration.
Hydrophobic effect
The driving mechanism for micellization is the transfer of hydrocarbon chains from water into the oil-like interior. This entropic effect is called hydrophobic effect. Compared to the increase of entropy of the surrounding water molecules, this hydrophobic interaction is relatively small. The water molecules are highly ordered around the hydrocarbon chain. The CMC decreases while the length of the alkyl chain increases when all the hydrocarbon chains are hidden inside micelles.[2] This can be modeled as a one step chemical process with the equation ,[3] where NS is the number of surfactant monomers in solution that associate into a micelle M.
Using this model, an equation can be derived for the Gibbs free energy.
where is the molar Gibbs energy of micellization, is the universal gas constant, is the absolute temperature, is the aggregation number (monomers per micelle), is the concentration of micelles, and is the critical micelle concentration. If is sufficiently large, the first term can be approximated as zero and the equation reduces to the general one listed at the start of this section.
Head group repulsion
The driving force for adsorption is the attraction between the surface and the surfactant head-group with low surfactant concentrations and the adsorption on hydrophilic surfaces. This means that the surfactant adsorbs at low surfactant concentrations with its head-group contacting the surface. Depending on the type of head-group and surface, the attraction will have a short-range contribution for both non-ionic and ionic surfactants. Ionic surfactants will also experience a generic electrostatic interaction. If the surfactants and the surface are oppositely charged than the interaction will be attractive.[2] If the surfactants and the surface are like charges than the interaction will be repulsive.[2] Aggregation is opposed due to the repulsion of the polar head groups as they come closer to each other. Hydration repulsion occurs because head groups have to be dehydrated as they come closer to each other.[2] The head groups’ thermal fluctuations become smaller as they come closer together because they are confined by neighboring head groups.[2] This causes their entropy to decrease and leads to a repulsion.[2]
Effect of increasing salt concentration
Near the CMC, aliphatic segments replace the head group segments from the surface because the affinity of the tail segments to the surface is so strong when there is a low salt concentration.[2] The surface charge adjusts itself to the surfactant adsorption when the salt concentration is low.[2] This results in a decrease of the surface charge at high coverages. When the salt concentration is high, the surface charge adjustment is weak. When at high salt concentration, the head segments get replaced by the tail segment during the last part of the isotherm.[2] The surface charge is barely adjusted when the salt concentration is high. There is also a competition between the surfactant ions and the salt ions. This leads to the surface charge adaptation vanishing. A much higher final surfactant adsorption occurs at high salt concentration than at a low salt concentration. This is due to the effective screening of the headgroup repulsion.[2] To account for this effect, the counterion can be included in the chemical equation to give:
[3] where NS is the number of surfactant monomers in solution that aggregate into micelle M, and I is the counterion that binds to the molecule. Once again, a Gibbs free energy can be derived from this, yielding:
where is the molar Gibbs energy of micellization, is the universal gas constant, is the absolute temperature, is the molar fraction of surfactant in solution, and is the molar fraction of surfactant in micelles. At the critical micelle concentration, the second term in the equation can be approximated as zero and the equation once again reduces to that given at the start of this section.
Dressed micelle model
In the dressed micelle model, the total Gibbs energy is broken down into several components accounting for the hydrophobic tail, the electrostatic repulsion of the head groups, and the interfacial energy on the surface of the micelle.
where the components of the total Gibbs micellization energy are hydrophobic, electrostatic, and interfacial.
Effect of concentration and temperature
Solubility and cloud point
Specific temperature at a specific pressure at which large groups of micelles begin to precipitate out into a quasi-separate phase.[5] As temperature is raised above the cloud point this causes the distinct surfactant phase to form densely packed micelle groups known as aggregates.[5] The phase separation is a reversible separation controlled by enthalpy (promotes aggregation/separation) above the cloud point, and entropy (promotes miscibility of micelles in water) below the cloud point. The cloud point is the equilibrium between the two free energies.[5]
Critical micelle concentration
The critical micelle concentration (CMC) is the exact concentration of surfactants at which aggregates become thermodynamically soluble in an aqueous solution. Below the CMC there is not a high enough density of surfactant to spontaneously precipitate into a distinct phase.[6] Above the CMC, the solubility of the surfactant within the aqueous solution has been exceeded. The energy required to keep the surfactant in solution no longer is the lowest energy state. To decrease free energy of the system the surfactant is precipitated out. CMC is determined by establishing inflection points for pre-determined surface tension of surfactants in solution. Plotting the inflection point against the surfactant concentration will provide insight into the critical micelle concentration by showing stabilization of phases.[6]
Krafft temperature
The Krafft temperature is the temperature at which the CMC can be achieved. This temperature determines the relative solubility of surfactant in an aqueous solution. This is the minimum temperature the solution must be at to allow the surfactant to precipitate into aggregates.[7] Below this temperature no level of solubility will be sufficient to precipitate aggregates due to minimal movement of particles in solution.[7] The Krafft Temperature (Tk) is based on the concentration of counter-ions (Caq).[7] Counter-ions are typically in the form of salt. Because the Tk is fundamentally based on the Caq, which is controlled by surfactant and salt concentration, different combinations of the respective parameters can be altered.[7] Although, the Caq will maintain the same value despite changes in concentration of surfactant and salt, therefore, thermodynamically speaking the Krafft temperature will remain constant.[7]
Surfactant packing parameter
Differences in shape
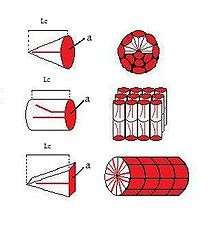
The shape of a surfactant molecule can be described by its surfactant packing parameter, Ns.[8] The packing parameter takes into account the volume of the hydrophobic chain (Vc), the cross sectional area of the hydrophilic core of the aggregate expressed per molecule in the aggregate (a), and the length of the hydrophobic chain (Lc):[8]
It should also be noted that the packing parameter for a specific surfactant is not a constant. It is dependent on various conditions which effect each the volume of the hydrophobic chain, the cross sectional area of the hydrophilic head group, and the length of the hydrophobic chain. Things that can affect these include, but are not limited to, the properties of the solvent, the solvent temperature, and the ionic strength of the solvent.
Cone, wedge, and cylinder shaped surfactants
The shape of a micelle is directly dependent on the packing parameter of the surfactant. Surfactants with a packing parameter of Ns ≤ 1/3 appear to have a cone-like shape which will pack together to form spherical micelles when in an aqueous environment (top in figure).[8][9] Surfactants with a packing parameter of 1/3 < Ns ≤ 1/2 appear to have a wedge-like shape and will aggregate together in an aqueous environment to form cylindrical micelles (bottom in figure).[8][9] Surfactants with a packing parameter of Ns > 1/2 appear to have a cylindrical shape and pack together to from a bilayer in an aqueous environment (middle in figure).[8][9]
Data
| Surfactant | Structure | CMC (mM) | ΔG (kJ/mol) |
|---|---|---|---|
| Sodium Dodecyl Sulfate (SDS) | 8.2[10] | -22.00 | |
| Sodium Octyl Sulfate (SOS) |  |
-- | -14.71 |
| Cetyl Trimethylammonium Bromide (CTAB) | 0.89−0.93 [11] | -30.46[12] |
References
- 1 2 3 4 Butt, Graf, Kappl (2006). Physics and Chemistry of Interfaces. Weinheim: Wiley-VCH. pp. 269–277. ISBN 978-3-527-40629-6.
- 1 2 3 4 5 6 7 8 9 10 Esumi, Kunio, and Minoru Ueno. Structure-performance Relationships in Surfactants. 2nd ed. Vol. 122. New York: Marcel Dekker, 2003.
- 1 2 3 4 Jones, Chapman (1995). Micelles, Monolayers, and Biomembranes. New York: Wiley-Liss. pp. 89–95. ISBN 0-471-56139-8.
- ↑ Stokes, Evans (1997). Fundamentals of Interfacial Engineering. New York: Wiley and Sons. p. 222. ISBN 978-0-471-18647-2.
- 1 2 3 Paleologos, Evangelos K.; Giokas, Dimosthenis L.; Karayannis, Miltiades I. (2005). "Micelle-mediated separation and cloud-point extraction". TrAC Trends in Analytical Chemistry. 24 (5): 426–436. doi:10.1016/j.trac.2005.01.013.
- 1 2 Daniel E. Klle, Cary 1. Chlou, Water Solubility Enhancements of DDT and Trichlorobenzene by Some Surfactants Below and Above the Critical Micelle Concentration, Environ. Sci Technol. 1989, 23, 832-838
- 1 2 3 4 5 Carolina Vautier-Giongo, Barney L. Bales, Estimate of the Ionization Degree of Ionic Micelles Based on Krafft Temperature Measurements, J. Phys. Chem. B 2003, 107, 5398-5403
- 1 2 3 4 5 Cullis, Pieter (1986). "Lipid Polymorphism and the Roles of Lipids in Membranes". Chemistry and Physics of Lipids. 40: 127–144. doi:10.1016/0009-3084(86)90067-8.
- 1 2 3 Borsali, Pecora (2008). Soft-Matter Characterization. Springer. p. 195. ISBN 978-1402044649.
- ↑ P. Mukerjee and K. J. Mysels, "Critical Micelle Concentration of Aqueous Surfactant Systems", NSRDS-NBS 36, US. Government Printing Office, Washington, D.C., 197 1.
- ↑ Wang, Yaofeng (2009). "Encapsulation of Myoglobin in a Cetyl Trimethylammonium Bromide Micelle in Vacuo: A Simulation Study". Biochemistry. 48 (5): 1006–1015. doi:10.1021/bi801952f.
- ↑ Rodriguez, Amalia (2003). "Water-Ethylene Glycol Alkyltrimethylammonium Bromide Micellar Solutions as Reaction Media: Study of Spontaneous Hydrolysis of Phenyl Chloroformate.". Langmuir. 19: 7206–7213. doi:10.1021/la0301137.