Tolman electronic parameter
3L.png)
The Tolman electronic parameter (TEP), named after Chadwick A. Tolman, is a measure of the electron donating or withdrawing ability of a ligand. It is determined by measuring the frequency of the A1 C-O vibrational mode of a complex, LNi(CO)3 by infrared spectroscopy, where L is the ligand being studied. LNi(CO)3 was chosen as the model compound because such complexes are readily prepared from tetracarbonylnickel(0).[1][2]
The carbonyl band is quite distinctive, and is rarely obscured by other bands in the analyte's infrared spectrum. Carbonyl is a small ligand so steric factors do not complicate the analysis.
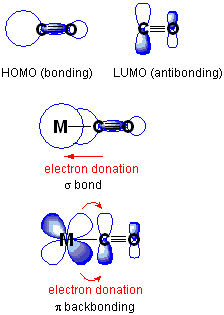
Upon coordination to a metal, ν(CO) typically decreases from 2143 cm−1 of free CO. This can be explained by π backbonding: the metal is able to form a π bond with the carbonyl ligand by donating electrons through its d orbitals into the empty π* anti-bonding orbitals on CO. This strengthens the metal-carbon bond, but also weakens the carbon-oxygen bond. If other ligands increase the density of π electrons on the metal, the C-O bond is weakened and ν(CO) decreases; conversely, if other ligands compete with CO for π backbonding, ν(CO) increases.
| L | ν(CO) cm−1 |
|---|---|
| P(t-Bu)3 | 2056.1 |
| PMe3 | 2064.1 |
| PPh3 | 2068.9 |
| P(OEt)3 | 2076.3 |
| PCl3 | 2097.0 |
| PF3 | 2110.8 |
The Tolman cone angle and TEP has been used to characterize the steric and electronic properties of phosphines, which are popular ligands for catalysts.
See also
References
- ↑ Robert H. Crabtree (2005). Carbonyls, Phosphine Complexes, and Ligand Substitution Reactions. pp. 87–124. doi:10.1002/0471718769.ch4.
- 1 2 Tolman, C. A. (1977). "Steric effects of phosphorus ligands in organometallic chemistry and homogeneous catalysis". Chem. Rev. 77 (3): 313–348. doi:10.1021/cr60307a002.
Further reading
- Tonner, Ralf; Frenking, Gernot (2009). "Tolman's Electronic Parameters for Divalent Carbon(0) Compounds". Organometallics. 28 (13): 3901–3905. doi:10.1021/om900206w.
- Gusev, Dmitry G. (2009). "Electronic and Steric Parameters of 76 N-Heterocyclic Carbenes in Ni(CO)3(NHC)". Organometallics. 28 (22): 6458–6461. doi:10.1021/om900654g.
3L.png)