Triazabicyclodecene
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
3,4,6,7,8,9-Hexahydro-2H-pyrimido[1,2-a]pyrimidine | |||
| Other names
1,3,5-Triazabicyclo[4.4.0]dec-5-ene TBD Hexahydropyrimidopyrimidine hpp | |||
| Identifiers | |||
| 5807-14-7 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 72164 | ||
| ECHA InfoCard | 100.024.880 | ||
| PubChem | 79873 | ||
| |||
| |||
| Properties | |||
| C7H13N3 | |||
| Molar mass | 139.20 g/mol | ||
| Melting point | 125 to 130 °C (257 to 266 °F; 398 to 403 K) | ||
| Acidity (pKa) | 15.2 ± 1.0[2] (pKa of conjugate acid in water); 26.03[3] (pKa of conjugate acid in acetonitrile) | ||
| Hazards | |||
| R-phrases | R34 | ||
| S-phrases | S26 S36/37/39 S45 | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Triazabicyclodecene (1,3,5-Triazabicyclo[4.4.0]dec-5-ene or TBD) is a commercially available bicyclic strong guanidine base (pKa = 25.98 in CH3CN and pKa = 21.00 in THF). TBD is an organic soluble base which has been used effectively for a variety of base-mediated organic transformations such as:[4]
- Michael reactions,
- Henry (nitroaldol reactions),
- Wittig reactions,
- Horner–Wadsworth–Emmons reactions,
- transesterification reactions,
- etherifications,
- ring-opening polymerizations (Scheme 1),
- tautomerizations and epimerizations
- P–C and P–N bond formations,
- Knoevenagel condensations,
- deprotonation reactions of phenols, carboxylic acids and C-acids (Scheme 2),
- aminolysis of esters.[5]
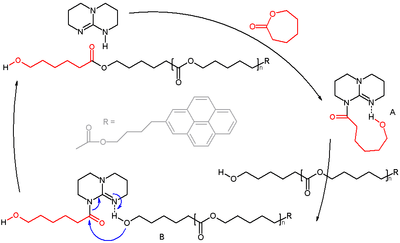
Scheme 1. Caprolactone polymerization. TBD is used as a catalyst in the ring opening polymerization of caprolactone to polycaprolactone. TBD acts as a bifunctional catalyst with simultaneous acyl transfer and hydrogen bonding.[6][7]
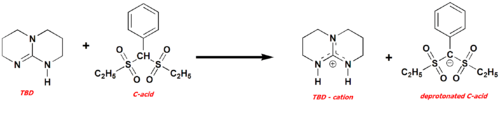
Scheme 2. Deprotonation of C–H acid. TBD can be considered as a very interesting agent for studies of the proton transfer reaction reactions and formation of the hydrogen-bonded chains with phenols and C–H acids.[8]
It is also known in deprotonated form (at the 7-position), often as a ligand, for instance in Ditungsten tetra(hpp)
References
- ↑ 1,5,7-Triazabicyclo[4.4.0]dec-5-ene at Sigma-Aldrich
- ↑ Kaupmees, K.; Trummal, A.; Leito, I. (2014). "Basicities of Strong Bases in Water: A Computational Study". Croat. Chem. Acta. 87: 385–395. doi:10.5562/cca2472.
- ↑ Kaljurand, I.; Kütt, A.; Sooväli, L.; Rodima, T.; Mäemets, V.; Leito, I.; Koppel, I. A. (2005). "Extension of the Self-Consistent Spectrophotometric Basicity Scale in Acetonitrile to a Full Span of 28 pKa Units: Unification of Different Basicity Scales". J. Org. Chem. 70: 1019–1028. doi:10.1021/jo048252w. PMID 15675863.
- ↑ Huczynski, Adam; Brzezinski, Bogumil (2008). "1,5,7-Triazabicyclo[4.4.0]dec-5-ene". e-EROS Encyclopedia of Reagents for Organic Synthesis. John Wiley & Sons. doi:10.1002/047084289X.rn00786.
- ↑ Sabot, Cyrille; Kumar, Kanduluru Ananda; Meunier, Stéphane; Mioskowski, Charles (2007). "A convenient aminolysis of esters catalyzed by 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD) under solvent-free conditions". Tetrahedron Lett.: 3863–3866. doi:10.1016/j.tetlet.2007.03.146.
- ↑ Pratt, Russell C.; Lohmeijer, Bas G. G.; Long, David A.; Waymouth, Robert M.; Hedrick, James L. (2006). "Triazabicyclodecene: A Simple Bifunctional Organocatalyst for Acyl Transfer and Ring-Opening Polymerization of Cyclic Esters". J. Am. Chem. Soc. 128 (14): 4556–4557. doi:10.1021/ja060662+.
- ↑ Reaction specs: initiator 4-pyrenebutanol (pyrene enables end-group determination by UV–vis) and monomer caprolactone added in ratio 1:100, targeted degree of polymerization = 100, with TBD cat. 0.5% in benzene; 72% conversion in 8 hours; polydispersity index 1.16
- ↑ Huczyński, A.; Binkowska, I.; Jarczewski, A.; Brzezinski, B. (2007). "Spectroscopic studies of the 1:1 complexes of 4-nitrophenyl(bis(ethylsulfonyl))methane and phenyl(bis(ethylsulfonyl))methane with 7-methyl-1,5,7-triazabicyclo(4.4.0)dec-5-ene and 1,5,7-triazabicyclo(4.4.0)dec-5-ene". J. Mol. Struc. 841 (1–3): 133–136. doi:10.1016/j.molstruc.2007.01.005.
This article is issued from Wikipedia - version of the 10/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
.png)