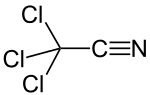
Trichloroacetonitrile
 | |
| Names | |
|---|---|
| IUPAC name
Trichloroacetonitrile | |
| Identifiers | |
| 545-06-2 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.008.078 |
| PubChem | 24900271 |
| |
| Properties | |
| C2Cl3N | |
| Molar mass | 144.38 g·mol−1 |
| Appearance | colourless liquid |
| Density | 1.44 g/mL |
| Melting point | -42 |
| Boiling point | 83 to 84 °C (181 to 183 °F; 356 to 357 K) |
| insoluble | |
| Hazards | |
| Main hazards | GHS06, GHS09 |
| Safety data sheet | MSDS |
| NFPA 704 | |
| Flash point | 195 °C (383 °F; 468 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Trichloroacetonitrile is an organic compound with the formula CCl3CN. It is a colourless liquid, although commercial samples often are brownish. It is used commercially as a precursor to the fungicide etridiazole. It is prepared by dehydration of trichloroacetamide.[1]
In academic research, trichloroacetonitrile is used as a reagent in the Overman rearrangement, converting allylic alcohols into allylic amines.[2][3][4]
See also
References
- ↑ Peter Pollak, Gérard Romeder, Ferdinand Hagedorn, Heinz-Peter Gelbke "Nitriles" in Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a17_363
- ↑ T. Nishikawa; M. Asai; N. Ohyabu; M. Isobe (1998). "Improved Conditions for Facile Overman Rearrangement(1)". J. Org. Chem. 63 (1): 188–192. doi:10.1021/jo9713924. PMID 11674062.
- ↑ "Overman Rearrangement". Organic Chemistry Portal. organic-chemistry.org. Retrieved November 15, 2012.
- ↑ Y. K. Chen. A. E. Lurain, P. J. Walsh (2002). "A general, highly enantioselective method for the synthesis of D and L alpha-amino acids and allylic amines". J. Am. Chem. Soc. 124 (41): 12225–12231. doi:10.1021/ja027271p. PMID 12371863.
This article is issued from Wikipedia - version of the 6/6/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
