Triose

Dihydroxyacetone is a ketotriose because the carbonyl group is the center of the chain.
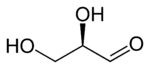
A triose is a monosaccharide, or simple sugar, containing three carbon atoms. There are only three possible trioses: L-Glyceraldehyde and D-Glyceraldehyde, both aldotrioses because the carbonyl group is at the end of the chain, and dihydroxyacetone, a ketotriose because the carbonyl group is the center the chain.[1]
Trioses are important in cellular respiration. During glycolysis, fructose-1,6-diphosphate is broken down into glyceraldehyde-3-phosphate and dihydroxyacetone phosphate. Lactic acid and pyruvic acid are later derived from these molecules.[2]
References
- ↑ "Trioses - Three Carbon Sugars". Oxford University Press. Retrieved 2011-07-10.
- ↑ "Glycolysis in Detail". Ohio State University at Mansfield. Retrieved 2011-07-10.
This article is issued from Wikipedia - version of the 8/9/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.