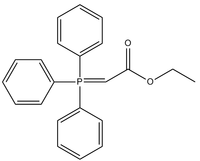
Triphenylcarbethoxymethylenephosphorane
 | |
| Names | |
|---|---|
| Other names
(2-Ethoxy-2-oxoethylidene)triphenylphosphorane (Carbethoxymethylene)triphenylphosphorane | |
| Identifiers | |
| 1099-45-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 63836 |
| ECHA InfoCard | 100.012.865 |
| PubChem | 24892754 |
| |
| |
| Properties | |
| C22H21O2P | |
| Molar mass | 348.38 g·mol−1 |
| Melting point | 124 to 129 °C (255 to 264 °F; 397 to 402 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Triphenylcarbethoxymethylenephosphorane is an organophosphorus compound with the chemical formula Ph3PCHCO2Et (Ph = phenyl, Et = ethyl). It is a white solid that is soluble in organic solvents.
The compound is a Wittig reagent. It is used to replace oxygen centres in ketones and aldehydes with CHCO2Et.[1]
References
- ↑ Lang, R. W.; Hansen, H.-J. (1984). "α-Allenic Esters from α-Phosphoranylidene Esters and Acid Chlorides: Ethyl 2,3-Pentadienoate" (PDF). Org. Synth. 62: 202.; Coll. Vol., 7, p. 232
This article is issued from Wikipedia - version of the 3/4/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.