ZnuABC
| High-affinity zinc uptake system protein ZnuA | |
|---|---|
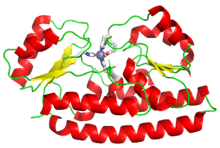 The structure of ZnuA bound to zinc (gray sphere), with coordinating amino acids shown in white. From PDB: 2OSV.[1] | |
| Identifiers | |
| Organism | |
| Symbol | ZnuA |
| PDB | 2OSV |
| UniProt | P39172 |
| High-affinity zinc uptake system membrane protein ZnuB | |
|---|---|
| Identifiers | |
| Organism | |
| Symbol | ZnuB |
| UniProt | P39832 |
| High-affinity zinc uptake system membrane protein ZnuC | |
|---|---|
| Identifiers | |
| Organism | |
| Symbol | ZnuC |
| UniProt | P0A9X1 |
ZnuABC is a high-affinity transporter specialized for transporting zinc ions as part of a system for metal ion homeostasis in bacteria. The complex is a member of the ATP-binding cassette (ABC) transporter protein family. The transporter contains three protein components:[2][3]
- ZnuA, a periplasmic zinc-binding protein.
- ZnuB, an integral membrane protein that transports zinc across the cytoplasmic membrane.
- ZnuC, an ATPase responsible for coupling ion transport to ATP hydrolysis.
The expression of ZnuABC is regulated by the zinc uptake regulator (Zur) protein and is induced by conditions of zinc starvation. Because zinc is often a limiting factor in bacterial infections, some pathogenic bacteria are heavily dependent on ZnuABC to scavenge zinc from the environment in an animal host.[4]
The periplasmic protein ZnuA interacts with ZinT, another component of the regulon controlled by Zur, which is also involved in periplasmic zinc homeostasis.[4][5][6][7]
References
- ↑ Li, H; Jogl, G (18 May 2007). "Crystal structure of the zinc-binding transport protein ZnuA from Escherichia coli reveals an unexpected variation in metal coordination.". Journal of Molecular Biology. 368 (5): 1358–66. doi:10.1016/j.jmb.2007.02.107. PMID 17399739.
- ↑ Patzer, SI; Hantke, K (June 1998). "The ZnuABC high-affinity zinc uptake system and its regulator Zur in Escherichia coli.". Molecular Microbiology. 28 (6): 1199–210. doi:10.1046/j.1365-2958.1998.00883.x. PMID 9680209.
- ↑ Yatsunyk, LA; Easton, JA; Kim, LR; Sugarbaker, SA; Bennett, B; Breece, RM; Vorontsov, II; Tierney, DL; Crowder, MW; Rosenzweig, AC (February 2008). "Structure and metal binding properties of ZnuA, a periplasmic zinc transporter from Escherichia coli.". Journal of Biological Inorganic Chemistry. 13 (2): 271–88. doi:10.1007/s00775-007-0320-0. PMC 2630496
 . PMID 18027003.
. PMID 18027003. - 1 2 Gabbianelli, R; Scotti, R; Ammendola, S; Petrarca, P; Nicolini, L; Battistoni, A (21 February 2011). "Role of ZnuABC and ZinT in Escherichia coli O157:H7 zinc acquisition and interaction with epithelial cells.". BMC microbiology. 11: 36. doi:10.1186/1471-2180-11-36. PMC 3053223
 . PMID 21338480.
. PMID 21338480. - ↑ Ilari, A; Alaleona, F; Tria, G; Petrarca, P; Battistoni, A; Zamparelli, C; Verzili, D; Falconi, M; Chiancone, E (January 2014). "The Salmonella enterica ZinT structure, zinc affinity and interaction with the high-affinity uptake protein ZnuA provide insight into the management of periplasmic zinc.". Biochimica et Biophysica Acta. 1840 (1): 535–44. doi:10.1016/j.bbagen.2013.10.010. PMID 24128931.
- ↑ Petrarca, P; Ammendola, S; Pasquali, P; Battistoni, A (March 2010). "The Zur-regulated ZinT protein is an auxiliary component of the high-affinity ZnuABC zinc transporter that facilitates metal recruitment during severe zinc shortage.". Journal of Bacteriology. 192 (6): 1553–64. doi:10.1128/jb.01310-09. PMC 2832539
 . PMID 20097857.
. PMID 20097857. - ↑ Blindauer, CA (18 March 2015). "Advances in the molecular understanding of biological zinc transport.". Chemical communications (Cambridge, England). 51 (22): 4544–63. doi:10.1039/c4cc10174j. PMID 25627157.