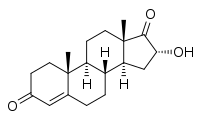
16α-Hydroxyandrostenedione
 | |
| Names | |
|---|---|
| IUPAC name
(8R,9S,10R,13S,14S,16R)-16-Hydroxy-10,13-dimethyl-2,6,7,8,9,11,12,14,15,16-decahydro-1H-cyclopenta[a]phenanthrene-3,17-dione | |
| Other names
16α-Hydroxyandrost-4-ene-3,17-dione; 16α-OH-A4 | |
| Identifiers | |
| 63-02-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27582 |
| ChEMBL | ChEMBL1908014 |
| ChemSpider | 389474 |
| PubChem | 440574 |
| UNII | JN8H7214IR |
| |
| |
| Properties | |
| C19H26O3 | |
| Molar mass | 302.414 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
16α-Hydroxyandrostenedione (16α-OH-A4), also known as 16α-hydroxyandrost-4-ene-3,17-dione, is an endogenous and naturally occurring steroid and metabolic intermediate in the biosynthesis of estriol during pregnancy.[1][2][3] It is produced from dehydroepiandrosterone (DHEA), which is converted into 16α-hydroxy-DHEA sulfate, then desulfated and aromatized into 16α-hydroxyestrone, and finally converted into estriol by 17β-hydroxysteroid dehydrogenase.[1][2]
See also
References
- 1 2 Rodney Rhoades; David R. Bell (2009). Medical Physiology: Principles for Clinical Medicine. Lippincott Williams & Wilkins. pp. 713–714. ISBN 978-0-7817-6852-8.
- 1 2 Charles Graham (2 December 2012). Reproductive Biology of the Great Apes: Comparative and Biomedical Perspectives. Elsevier. pp. 56–. ISBN 978-0-323-14971-6.
- ↑ Vitamins and Hormones. Academic Press. 7 September 2005. pp. 282–. ISBN 978-0-08-045978-3.
This article is issued from Wikipedia - version of the 12/3/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.