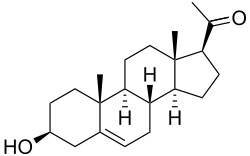
Pregnenolone
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral, transdermal |
| ATC code | None |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number |
145-13-1 |
| PubChem (CID) | 8955 |
| IUPHAR/BPS | 2376 |
| DrugBank |
DB02789 |
| ChemSpider |
8611 |
| UNII |
73R90F7MQ8 |
| ChEBI |
CHEBI:16581 |
| ChEMBL |
CHEMBL253363 |
| Chemical and physical data | |
| Formula | C21H32O2 |
| Molar mass | 316.483 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineralocorticoids. In addition, pregnenolone is biologically active in its own right, acting as a neurosteroid.[1]
Biochemistry
Biosynthesis
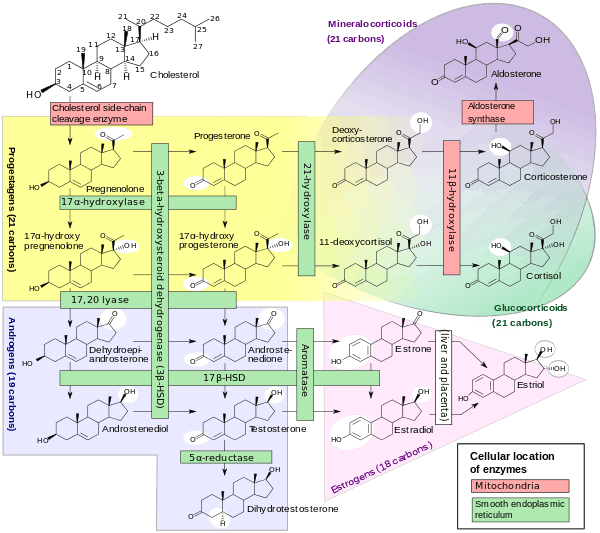
Pregnenolone is synthesized from cholesterol. This conversion involves hydroxylation of the side chain at the C20 and C22 positions, with cleavage of the side chain. The enzyme performing this task is cytochrome P450scc, located in the mitochondria, and controlled by anterior pituitary trophic hormones, such as adrenocorticotropic hormone, follicle-stimulating hormone, and luteinizing hormone, in the adrenal glands and gonads. There are two intermediates in the transformation of cholesterol into pregnenolone, 22R-hydroxycholesterol and 20α,22R-dihydroxycholesterol, and all three steps in the transformation are catalyzed by P450scc.
To assay conversion of cholesterol to pregnenolone, radiolabelled cholesterol has been used.[2] Pregnenolone product can be separated from cholesterol substrate using Sephadex LH-20 minicolumns.[2]
Pregnenolone is produced mainly in the gonads, adrenal glands, and the brain.[3]
Metabolism
Pregnenolone undergoes further steroid metabolism in one of several ways:
- Pregnenolone can be converted into progesterone. The critical enzyme step is two-fold using a 3β-hydroxysteroid dehydrogenase and a Δ5-4 isomerase. The latter transfers the double bond from C5 to C4 on the A ring. Progesterone is the entry into the Δ4 pathway, resulting in production of 17α-hydroxyprogesterone and androstenedione, precursor to testosterone and estrone. Aldosterone and corticosteroids are also derived from progesterone or its derivatives.
- Pregnenolone can be converted to 17α-hydroxypregnenolone by the enzyme 17α-hydroxylase (CYP17A1). Using this pathway, termed Δ5 pathway, the next step is conversion to dehydroepiandrosterone (DHEA) via 17,20-lyase (CYP17A1). DHEA is the precursor of androstenedione.
- Pregnenolone can be converted to androstadienol by 16-ene synthase (CYP17A1).
- Pregnenolone can be converted to pregnenolone sulfate by steroid sulfotransferase, and this conversion can be reversed by steroid sulfatase.
Biological activity
Neurosteroid activity
Pregnenolone and its 3β-sulfate, pregnenolone sulfate, like DHEA, DHEA sulfate, and progesterone, belong to the group of neurosteroids that are found in high concentrations in certain areas of the brain, and are synthesized there. Neurosteroids affect synaptic functioning, are neuroprotective, and enhance myelinization. Pregnenolone and its sulfate ester are under investigation for their potential to improve cognitive and memory functioning.[4] Pregnenolone is also being considered as a potential treatment for schizophrenia.[1]
Although pregnenolone itself does not possess these activities, its metabolite pregnenolone sulfate is a negative allosteric modulator of the GABAA receptor[5] as well as a positive allosteric modulator of the NMDA receptor.[6][7] In addition, pregnenolone sulfate has been shown to activate the transient receptor potential M3 (TRPM3) ion channel in hepatocytes and pancreatic islets causing calcium entry and subsequent insulin release.[8]
Pregnenolone is involved in a natural negative feedback loop against CB1 receptor activation in animals.[9] It prevents CB1 receptor agonists like tetrahydrocannabinol, the main active constituent in cannabis, from fully activating the CB1 receptor, that when stimulated by tetrahydrocannabinol causes the psychological effects of cannabis.[9]
Microtubule-associated protein 2
Pregnenolone has been found to bind with high, nanomolar affinity to microtubule-associated protein 2 (MAP2) in the brain.[10][11] In contrast to pregnenolone, pregnenolone sulfate did not bind to microtubules.[10][11] However, progesterone did and with similar affinity to pregnenolone, although unlike pregnenolone, it did not increase binding of MAP2 to tubulin.[10][11] Pregnenolone was found to induce tubule polymerization in neuronal cultures and to increase neurite growth in PC12 cells treated with nerve growth factor.[10][11] As such, pregnenolone may control formation and stabilization of microtubules in neurons and may affect both neural development during prenatal development and neural plasticity during aging.[10][11] The 3β-methyl ether of pregnenolone, 3β-methoxypregnenolone (MAP-4343), retains similar activity to pregnenolone in regards to interaction with MAP2,[10][11] and is under development for potential clinical use for indications such as the treatment of brain and spinal cord injury and depressive disorders.[12][13][14][15]
Nuclear receptors
Pregnenolone has been found to act as an agonist of the pregnane X receptor.[16]
Pharmacology
Oral administration of 50 or 100 mg pregnenolone has been found to have minimal or negligible effect on urinary levels of testosterone and testosterone metabolites, including of androsterone, etiocholanolone, 5β-androstanediol, androstadienol, and androstenol (and/or their conjugates), and this suggests that only a small amount of pregnenolone is converted into testosterone.[17][18] This is in accordance with findings on the conversion of DHEA into testosterone, in which only 1.5% of an oral dose of DHEA was found to be converted into testosterone.[17] In contrast to the androstanes, 50 or 100 mg oral pregnenolone has been found to significantly and in fact "strongly" increase urinary levels of the progesterone metabolites pregnanediol and pregnanolone (and/or their conjugates), whereas pregnanetriol was unaffected.[17][18] Unlike the case of oral administration, transdermal administration of 30 mg/day pregnenolone cream has not been found to affect urinary levels of metabolites of any other steroids, including of progesterone.[18]
Sripada et al. reported that oral pregnenolone is preferentially metabolized into the neurosteroid allopregnanolone rather than into other steroids such as DHEA or cortisol.[19] In further research by their group, a single 400 mg dose of oral pregnenolone at 3 hours post-administration was found to result in a 3-fold elevation in serum levels of pregnenolone and a 7-fold increase in allopregnanolone levels.[19] Pregnanolone levels increased by approximately 60% while DHEA levels decreased non-significantly by approximately 5% and cortisol levels were not affected.[19] Another study found that allopregnanolone levels were increased by 3-fold at 2 hours post-administration following a single 400 mg oral dose of pregnenolone.[19]
In addition to allopregnanolone, exogenous pregnenolone also functions as a prohormone of pregnenolone sulfate.[20]
Pregnenolone is lipophilic and readily crosses the blood-brain-barrier.[19]
Chemistry
Pregnenolone is also known chemically as pregn-5-en-3β-ol-20-one. Like other steroids, it consists of four interconnected cyclic hydrocarbons. The compound contains ketone and hydroxyl functional groups, two methyl branches, and a double bond at C5, in the B cyclic hydrocarbon ring. Like many steroid hormones, it is hydrophobic. The sulfated derivative, pregnenolone sulfate, is water-soluble.
3β-Dihydroprogesterone (pregn-4-en-3β-ol-20-one) is an isomer of pregnenolone in which the C5 double bond has been replaced with a C4 double bond.
References
- 1 2 Marx CE, Bradford DW, Hamer RM, et al. (September 2011). "Pregnenolone as a novel therapeutic candidate in schizophrenia: emerging preclinical and clinical evidence". Neuroscience. 191: 78–90. doi:10.1016/j.neuroscience.2011.06.076. PMID 21756978.
- 1 2 Hanukoglu I, Jefcoate CR (1980). "Pregnenolone separation from cholesterol using Sephadex LH-20 mini-columns". Journal of Chromatography A. 190 (1): 256–262. doi:10.1016/S0021-9673(00)85545-4.
- ↑ Vallée M (2016). "Neurosteroids and potential therapeutics: Focus on pregnenolone". J. Steroid Biochem. Mol. Biol. 160: 78–87. doi:10.1016/j.jsbmb.2015.09.030. PMID 26433186.
- ↑ Vallée M, Mayo W, Le Moal M (November 2001). "Role of pregnenolone, dehydroepiandrosterone and their sulfate esters on learning and memory in cognitive aging". Brain Research. Brain Research Reviews. 37 (1-3): 301–12. doi:10.1016/S0165-0173(01)00135-7. PMID 11744095.
- ↑ Majewska MD, Mienville JM, Vicini S (August 1988). "Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons". Neuroscience Letters. 90 (3): 279–84. doi:10.1016/0304-3940(88)90202-9. PMID 3138576.
- ↑ Wu FS, Gibbs TT, Farb DH (September 1991). "Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-D-aspartate receptor". Molecular Pharmacology. 40 (3): 333–6. PMID 1654510.
- ↑ Irwin RP, Maragakis NJ, Rogawski MA, Purdy RH, Farb DH, Paul SM (July 1992). "Pregnenolone sulfate augments NMDA receptor mediated increases in intracellular Ca2+ in cultured rat hippocampal neurons". Neurosci Lett. 141 (1): 30–4. doi:10.1016/0304-3940(92)90327-4. PMID 1387199.
- ↑ Wagner TF, Loch S, Lambert S, et al. (December 2008). "Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic beta cells". Nature Cell Biology. 10 (12): 1421–30. doi:10.1038/ncb1801. PMID 18978782.
- 1 2 http://www.sciencedaily.com/releases/2014/01/140102142012.htm
- 1 2 3 4 5 6 Mellon SH (2007). "Neurosteroid regulation of central nervous system development". Pharmacol. Ther. 116 (1): 107–24. doi:10.1016/j.pharmthera.2007.04.011. PMC 2386997
 . PMID 17651807.
. PMID 17651807. - 1 2 3 4 5 6 Fontaine-Lenoir V, Chambraud B, Fellous A, David S, Duchossoy Y, Baulieu EE, Robel P (2006). "Microtubule-associated protein 2 (MAP2) is a neurosteroid receptor". Proc. Natl. Acad. Sci. U.S.A. 103 (12): 4711–6. doi:10.1073/pnas.0600113103. PMC 1450236
 . PMID 16537405.
. PMID 16537405. - ↑ http://adisinsight.springer.com/drugs/800034216
- ↑ Duchossoy Y, David S, Baulieu EE, Robel P (2011). "Treatment of experimental spinal cord injury with 3β-methoxy-pregnenolone". Brain Res. 1403: 57–66. doi:10.1016/j.brainres.2011.05.065. PMID 21704982.
- ↑ Bianchi M, Baulieu EE (2012). "3β-Methoxy-pregnenolone (MAP4343) as an innovative therapeutic approach for depressive disorders". Proc. Natl. Acad. Sci. U.S.A. 109 (5): 1713–8. doi:10.1073/pnas.1121485109. PMC 3277154
 . PMID 22307636.
. PMID 22307636. - ↑ Baulieu É (2015). "From steroid hormones to depressive states and senile dementias: New mechanistic, therapeutical and predictive approaches". C. R. Biol. 338 (8-9): 613–6. doi:10.1016/j.crvi.2015.06.003. PMID 26251072.
- ↑ Kliewer SA, Lehmann JM, Milburn MV, Willson TM (1999). "The PPARs and PXRs: nuclear xenobiotic receptors that define novel hormone signaling pathways". Recent Prog. Horm. Res. 54: 345–67; discussion 367–8. PMID 10548883.
- 1 2 3 Saudan C, Desmarchelier A, Sottas PE, Mangin P, Saugy M (2005). "Urinary marker of oral pregnenolone administration". Steroids. 70 (3): 179–83. doi:10.1016/j.steroids.2004.12.007. PMID 15763596.
- 1 2 3 Piper T, Schlug C, Mareck U, Schänzer W (2011). "Investigations on changes in ¹³C/¹²C ratios of endogenous urinary steroids after pregnenolone administration". Drug Test Anal. 3 (5): 283–90. doi:10.1002/dta.281. PMID 21538944.
- 1 2 3 4 5 Sripada RK, Marx CE, King AP, Rampton JC, Ho SS, Liberzon I (2013). "Allopregnanolone elevations following pregnenolone administration are associated with enhanced activation of emotion regulation neurocircuits". Biol. Psychiatry. 73 (11): 1045–53. doi:10.1016/j.biopsych.2012.12.008. PMC 3648625
 . PMID 23348009.
. PMID 23348009. - ↑ Ducharme N, Banks WA, Morley JE, Robinson SM, Niehoff ML, Mattern C, Farr SA (2010). "Brain distribution and behavioral effects of progesterone and pregnenolone after intranasal or intravenous administration". Eur. J. Pharmacol. 641 (2-3): 128–34. doi:10.1016/j.ejphar.2010.05.033. PMC 3008321
 . PMID 20570588.
. PMID 20570588.