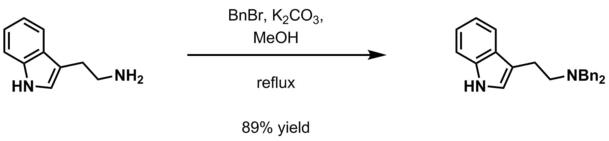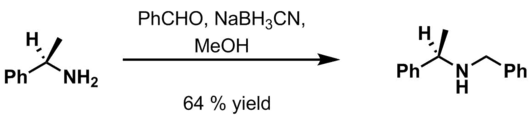Benzyl group
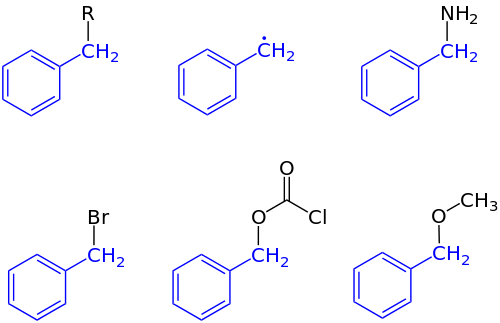
In organic chemistry, benzyl is the substituent or molecular fragment possessing the structure C6H5CH2–. Benzyl features a benzene ring attached to a CH2 group.[1]
Nomenclature
In IUPAC nomenclature the prefix benzyl refers to a C6H5CH2 substituent, for example benzyl chloride or benzyl benzoate. Benzyl is not to be confused with phenyl with the formula C6H5.
The term benzylic is used to describe the position of the first carbon bonded to a benzene or other aromatic ring. For example, the molecule, is referred to as a "benzylic" carbocation. The benzyl free radical has the formula C
6H
5CH•
2. The benzylium carbocation has the formula C
6H
5CH+
2; the carbanion has the formula C
6H
5CH−
2. None of these species can be formed in significant amounts under normal conditions, but they are useful referents for discussion of reaction mechanisms.
Abbreviations
The abbreviation "Bn" is frequently used to denote benzyl groups in nomenclature and structural depictions of chemical compounds. For example, benzyl alcohol can be represented as BnOH. This abbreviation is not to be confused with "Bz", which is the abbreviation for the benzoyl group C6H5C(O)−, or the phenyl group C6H5, abbreviated "Ph".
Reactivity of benzylic centers
The enhanced reactivity of benzylic positions is attributed to the low bond dissociation energy for benzylic C−H bonds. Specifically, the bond C6H5CH2−H is about 10–15% weaker than other kinds of C−H bonds. The neighboring aromatic ring stabilizes benzyl radicals. The data tabulated below compare benzylic C−H bond to related C−H bond strengths.
| Bond | Bond | Bond-dissociation energy | Comment | |
|---|---|---|---|---|
| (kcal/mol) | (kJ/mol) | |||
| C6H5CH2−H | benzylic C−H bond | 90 | 377 | akin to allylic C−H bonds such bonds show enhanced reactivity |
| H3C−H | Methyl C−H bond | 105 | 439 | One of the strongest aliphatic C−H bonds |
| C2H5−H | Ethyl C−H bond | 101 | 423 | slightly weaker than H3C−H |
| C6H5−H | phenyl C−H bond | 113 | 473 | comparable to vinyl radical, rare |
| CH2=CHCH2−H | allylic C–H bond | 89 | 372 | such bonds show enhanced reactivity |
The weakness of the C−H bond reflects the stability of the benzylic radical. For related reasons, benzylic substituents exhibit enhanced reactivity, as in oxidation, free radical halogenation, or hydrogenolysis. As a practical example, in the presence of suitable catalysts, p-xylene oxidizes exclusively at the benzylic positions to give terephthalic acid:
- CH3C6H4CH3 + 3 O2 → HO2CC6H4CO2H + 2 H2O.
Millions of tonnes of terephthalic acid are produced annually by this method.[2]
As a protecting group
Alcohol protection
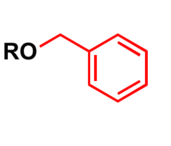
Benzyl, abbreviated as Bn, is commonly used in organic synthesis as a robust protecting group for alcohols and carboxylic acids.
Most common protection methods
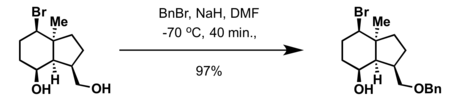
- Treatment of alcohol with a strong base such as powdered potassium hydroxide or sodium hydride and benzyl halide (BnCl or BnBr)[3][4]
- Monobenzylation of diols can be achieved using Ag2O in dimethylformamide (DMF) at ambient to elevated temperatures[5]
- Primary alcohols can be selectively benzylated in presence of phenol functional groups using Cu(acac)2[6]
Most common deprotection methods
Benzyl ethers can be removed under reductive conditions, oxidative conditions, and the use of Lewis Acids.[3]
Reductive conditions
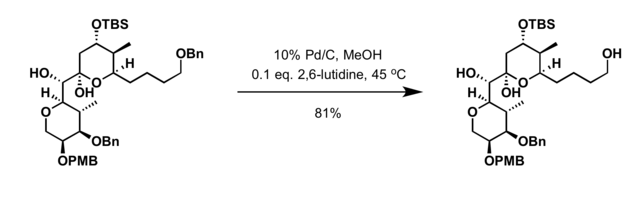
- Removed using hydrogenolysis[7]
- Single electron process with Na/NH3 or Li/NH3
Oxidative conditions
- Benzyl protecting groups can be removed using a wide range of oxidizing agents including:
- CrO3/acetic acid at ambient temperature
- Ozone
- N-Bromosuccinimide (NBS)
- N-Iodosuccinimide (NIS)
Lewis acid-based
- Trimethylsilyl iodide (Me3SiI) in dichloromethane at ambient temperature (selectivity can be achieved under specific conditions)
The p-methoxybenzyl protecting group
p-Methoxybenzyl (PMB) is used as a protecting group for alcohols in organic synthesis.
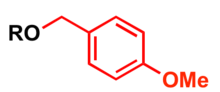
Most common protection methods
- Strong base such as powdered potassium hydroxide or sodium hydride and p-methoxybenzyl halide (chloride or bromide)[8][9]
- 4-methoxybenzyl-2,2,2-trichloroacetimidate can be used to install the PMB group in presence of:
- Scandium (III) triflate (Sc(OTf)3) in toluene at 0 °C[10]
- Trifluoromethanesulfonic acid (TfOH) in dichloromethane at 0 °C[11]
Most common deprotection methods
- 2,3-Dichloro-5,6-dicyano-p-benzoquinone (DDQ)[12]
- Conditions for deprotection of benzyl group are applicable for cleavage of the PMB protecting group
Amine protection

The benzyl group is largely used as a protecting group for amines in organic synthesis.
Most common amine protection methods
- Aqueous potassium carbonate and benzyl halide (BnCl, BnBr) in methanol[13]
- Benzaldehyde, 6 M HCl and NaBH3CN in methanol[14]
Most common amine deprotection methods
- Hydrogenation in the presence of the palladium catalyst[15]
See also
References
- ↑ Carey, F. A.; Sundberg, R. J. (2008). Advanced Organic Chemistry, Part A: Structure and Mechanisms (5th ed.). New York, NY: Springer. pp. 806–808, 312–313. ISBN 9780387448978.
- ↑ Sheehan, Richard J. (2005), "Terephthalic Acid, Dimethyl Terephthalate, and Isophthalic Acid", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a26_193
- 1 2 Wuts, Peter G. M.; Greene, Theodora W. Greene's Protective Groups in Organic Synthesis (4th ed.). Wiley Online Library. doi:10.1002/0470053488.
- ↑ Fukuzawa, Akio; Sato, Hideaki; Masamune, Tadashi (1987-01-01). "Synthesis of (±)-prepinnaterpene, a bromoditerpene from the red alga Yamada". Tetrahedron Letters. 28 (37): 4303–4306. doi:10.1016/S0040-4039(00)96491-8.
- ↑ Van Hijfte, Luc; Little, R. Daniel (1985-10-01). "Intramolecular 1,3-diyl trapping reactions. A formal total synthesis of (±)-coriolin". The Journal of Organic Chemistry. 50 (20): 3940–3942. doi:10.1021/jo00220a058. ISSN 0022-3263.
- ↑ Sirkecioglu, Okan; Karliga, Bekir; Talinli, Naciye (2003-11-10). "Benzylation of alcohols by using bis[acetylacetonato]copper as catalyst". Tetrahedron Letters. 44 (46): 8483–8485. doi:10.1016/j.tetlet.2003.09.106.
- ↑ Smith, Amos B.; Zhu, Wenyu; Shirakami, Shohei; Sfouggatakis, Chris; Doughty, Victoria A.; Bennett, Clay S.; Sakamoto, Yasuharu (2003-03-01). "Total Synthesis of (+)-Spongistatin 1. An Effective Second-Generation Construction of an Advanced EF Wittig Salt, Fragment Union, and Final Elaboration". Organic Letters. 5 (5): 761–764. doi:10.1021/ol034037a. ISSN 1523-7060.
- ↑ Marco, José L.; Hueso-Rodríguez, Juan A. (1988-01-01). "Synthesis of optically pure 1-(3-furyl)-1,2-dihydroxyethane derivatives". Tetrahedron Letters. 29 (20): 2459–2462. doi:10.1016/S0040-4039(00)87907-1.
- ↑ Takaku, Hiroshi; Kamaike, Kazuo; Tsuchiya, Hiromichi (1984-01-01). "Oligonucleotide synthesis. Part 21. Synthesis of ribooligonucleotides using the 4-methoxybenzyl group as a new protecting group for the 2′-hydroxyl group". The Journal of Organic Chemistry. 49 (1): 51–56. doi:10.1021/jo00175a010. ISSN 0022-3263.
- ↑ Trost, Barry M.; Waser, Jerome; Meyer, Arndt (2007-11-01). "Total Synthesis of (−)-Pseudolaric Acid B". Journal of the American Chemical Society. 129 (47): 14556–14557. doi:10.1021/ja076165q. ISSN 0002-7863. PMC 2535803
 . PMID 17985906.
. PMID 17985906. - ↑ Mukaiyama, Teruaki; Shiina, Isamu; Iwadare, Hayato; Saitoh, Masahiro; Nishimura, Toshihiro; Ohkawa, Naoto; Sakoh, Hiroki; Nishimura, Koji; Tani, Yu-ichirou (1999-01-04). "Asymmetric Total Synthesis of Taxol\R". Chemistry – A European Journal. 5 (1): 121–161. doi:10.1002/(SICI)1521-3765(19990104)5:13.0.CO;2-O. ISSN 1521-3765.
- ↑ Hanessian, Stephen; Marcotte, Stéphane; Machaalani, Roger; Huang, Guobin (2003-11-01). "Total Synthesis and Structural Confirmation of Malayamycin A: A Novel Bicyclic C-Nucleoside from Streptomyces malaysiensis". Organic Letters. 5 (23): 4277–4280. doi:10.1021/ol030095k. ISSN 1523-7060.
- ↑ Kuehne, Martin E.; Xu, Feng (1993-12-01). "Total synthesis of strychnan and aspidospermatan alkaloids. 3. The total synthesis of (±)-strychnine". The Journal of Organic Chemistry. 58 (26): 7490–7497. doi:10.1021/jo00078a030. ISSN 0022-3263.
- ↑ Cain, Christian M.; Cousins, Richard P. C.; Coumbarides, Greg; Simpkins, Nigel S. (1990-01-01). "Asymmetric deprotonation of prochiral ketones using chiral lithium amide bases". Tetrahedron. 46 (2): 523–544. doi:10.1016/S0040-4020(01)85435-1.
- ↑ Zhou, Hao; Liao, Xuebin; Cook, James M. (2004-01-01). "Regiospecific, Enantiospecific Total Synthesis of the 12-Alkoxy-Substituted Indole Alkaloids, (+)-12-Methoxy-Na-methylvellosimine, (+)-12-Methoxyaffinisine, and (−)-Fuchsiaefoline". Organic Letters. 6 (2): 249–252. doi:10.1021/ol0362212. ISSN 1523-7060.
External links
 Chemistry portal
Chemistry portal Quotations related to Benzyl group at Wikiquote
Quotations related to Benzyl group at Wikiquote