Brugada syndrome
| Brugada syndrome | |
|---|---|
|
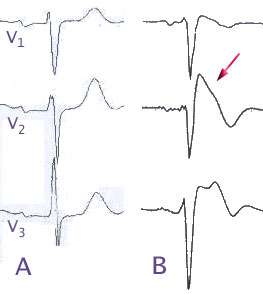
(A) Normal electrocardiogram pattern in the precordial leads V1-3, (B) changes in Brugada syndrome (type B) | |
| Classification and external resources | |
| Specialty | Cardiology |
| ICD-10 | I49.8 |
| ICD-9-CM | 746.89 |
| OMIM | 601144 |
| DiseasesDB | 31999 |
| eMedicine | med/3736 |
| MeSH | D053840 |
Brugada syndrome (BrS) is a genetic disease that is characterised by abnormal electrocardiogram (ECG) findings and an increased risk of sudden cardiac death. It is named for the Spanish cardiologists Pedro Brugada, Josep Brugada and Ramon Brugada. It is the major[1][2] cause of sudden unexplained death syndrome (SUDS), also known as sudden adult death syndrome (SADS), and is the most common cause of sudden death in young men without known underlying cardiac disease in Thailand and Laos.[3]
Although the ECG findings of Brugada syndrome were first reported[4] among survivors of cardiac arrest in 1989, it was only in 1992 that the Brugada brothers[5] recognized it as a distinct clinical entity, causing sudden death by causing ventricular fibrillation (a potentially lethal arrhythmia) in the heart.
Genetics
| Type | OMIM | Mutation | Notes |
|---|---|---|---|
| B1 | 601144 | SCN5A | alpha subunit of the sodium channel. Current through this channel is commonly referred to as INa. Loss of function in this channel leads to an unopposed Ito current (KCND2) |
| B2 | 611778 | GPD1L | Glycerol-3-phosphate dehydrogenase like peptide |
| B3 | 114205 | CACNA1C | Alpha subunit of cardiac L-type calcium channel.[6] |
| B4 | 600003 | CACNB2 | Beta-2 subunit of the voltage dependent L-type calcium channel.[6] |
| B5 | 604433 | KCNE3 which coassembles with KCND3 | Beta subunit to KCND3. Modulates the Ito potassium outward current[7] |
| B6 | 600235 | SCN1B | Beta-1 subunit of the sodium channel SCN5A[8] |
| SCN10A | [9] | ||
| HEY2 | [9] | ||
Approximately 20% of the cases of Brugada syndrome have been shown to be associated with mutations in a gene that encodes for a sodium ion channel in the cell membranes of the muscle cells of the heart (the myocytes); this is often referred to as a sodium channelopathy. The majority of patients affected by Brugada syndrome are not found to have known genetic mutations to explain the disease, as of 2015.[10] The gene, named SCN5A, is located on the short arm of the third chromosome (3p21). Loss-of-function mutations in this gene lead to a loss of the action potential dome of some epicardial areas of the right ventricle. This results in transmural and epicardial dispersion of repolarization. The transmural dispersion underlies ST-segment elevation and the development of a vulnerable window across the ventricular wall, whereas the epicardial dispersion of repolarization facilitates the development of phase 2 reentry, which generates a phase 2 reentrant extrasystole that captures the vulnerable window to precipitate ventricular tachycardia and/or ventricular fibrillation that often results in sudden cardiac death. At present time however, all the reported patients who died because of the disease and were submitted to detailed autopsy study have shown a structural right ventricular pathology underlying the syndrome.
Over 160 mutations in the SCN5A gene have been discovered to date, each having varying mechanisms and effects on function, thereby explaining the varying degrees of likelihood of the genetic mutation leading to the disease ( that is to say, penetrance) and expression of this disorder.[11]
An example of one of the mechanisms in which a loss of function of the sodium channel occurs is a mutation in the gene that disrupts the sodium channel's ability to bind properly to ankyrin-G, an important protein mediating interaction between ion channels and cytoskeletal elements. Very recently a mutation in a second gene, Glycerol-3-phosphate dehydrogenase 1-like gene (GPD1L) has been shown to result in Brugada syndrome in a large multigenerational family (London, 2006). This gene acts as an ion channel modulator in the heart, although the exact mechanism is not yet understood.
Recently Antzelevitch has identified mutations in the L-type calcium channel subunits (CACNA1C (A39V and G490R) and CACNB2 (S481L)) leading to ST elevation and a relatively short QT interval (below 360 ms).[12] For a comprehensive list of all mutations see [11] In 2013, Bezzina et al. showed that common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome.[9]
This condition is inherited in an autosomal dominant pattern and manifests itself more commonly in males, due to a higher penetrance. In addition it has a higher prevalence in most Asian populations.
Prevalence
The prevalence of Brugada ECG is indeed higher in Asia than in the United States and Europe. Specifically, Brugada Type 1 ECG appears more frequently in Asia (0%–0.36% of the population) and Europe (0%–0.25%) than in the United States (0.03%). Type 2 and Type 3 ECG is more prevalent in Asia (0.12%–2.23%) than in Europe (0.0%–0.6%) or the United States (0.02%).[13]
Diagnosis
Genetic testing for Brugada syndrome is clinically available and may help confirm a diagnosis, as well as differentiate between relatives who are at risk for the disease and those who are not. Some symptoms when pinpointing this disease include fainting, irregular heartbeats, and chaotic heartbeats. However, just detecting the irregular heartbeat may be a sign of another disease, so the doctor must detect another symptom as well.[14]
Electrocardiography
In some cases, the disease can be detected by observing characteristic patterns on an electrocardiogram. These patterns may be present all the time, they might be elicited by the administration of particular drugs (e.g., Class IA, such as ajmaline or procainamide, or class 1C, such as flecainide or pilsicainide, antiarrhythmic drugs that block sodium channels and cause appearance of ECG abnormalities), or they might resurface spontaneously due to as-yet unclarified triggers.
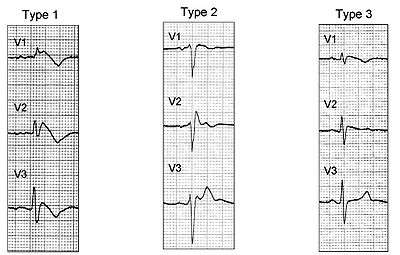
Brugada syndrome has three different ECG patterns:[15]
- Type 1 has a coved type ST elevation with at least 2 mm (0.2 mV) J-point elevation and a gradually descending ST segment followed by a negative T-wave.
- Type 2 has a saddle-back pattern with a least 2 mm J-point elevation and at least 1 mm ST elevation with a positive or biphasic T-wave. Type 2 pattern can occasionally be seen in healthy subjects.
- Type 3 has either a coved (type 1 like) or a saddle-back (type 2 like) pattern, with less than 2 mm J-point elevation and less than 1 mm ST elevation. Type 3 pattern is not rare in healthy subjects.
The pattern seen on the ECG is persistent ST elevations in the electrocardiographic leads V1-V3 with a right bundle branch block (RBBB) appearance, with or without the terminal S waves in the lateral leads that are associated with a typical RBBB. A prolongation of the PR interval (a conduction disturbance in the heart) is also frequently seen. The ECG can fluctuate over time, depending on the autonomic balance and the administration of antiarrhythmic drugs. Adrenergic stimulation decreases the ST segment elevation, while vagal stimulation worsens it. (There is a case report of a patient who died while shaving, presumed due to the vagal stimulation of the carotid sinus massage.)
The administration of class Ia, Ic, and III drugs increases the ST segment elevation, as does fever.[16] Exercise decreases ST segment elevation in some patients, but increases it in others (after exercise, when the body temperature has risen). The changes in heart rate induced by atrial pacing are accompanied by changes in the degree of ST segment elevation. When the heart rate decreases, the ST segment elevation increases, and when the heart rate increases, the ST segment elevation decreases. However, the contrary can also be observed.
Treatment
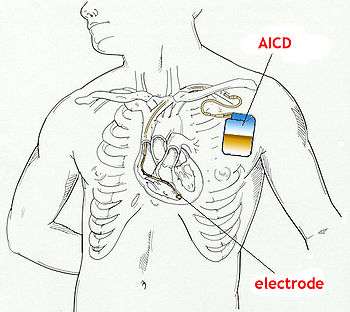
The cause of sudden death in Brugada syndrome is ventricular fibrillation (VF). The average age of death is 41. According to clinical reports, sudden death in people with Brugada syndrome most often happens during sleep. The episodes of syncope (fainting) and sudden death (aborted or not) are caused by fast polymorphic ventricular tachycardias or ventricular fibrillation. These arrhythmias appear with no warning. While there is no exact treatment modality that reliably and totally prevents ventricular fibrillation from occurring in this syndrome, treatment lies in termination of this lethal arrhythmia before it causes death. This is done via insertion of an implantable cardioverter-defibrillator (ICD), which continuously monitors the heart rhythm and will shock the wearer if ventricular fibrillation is sensed.
Recent studies have evaluated the role of quinidine, a Class Ia antiarrhythmic drug, for decreasing VF episodes occurring in this syndrome. Quinidine has been found to both decrease the number of VF episodes and correct spontaneous ECG changes, possibly via inhibiting Ito channels.[17] Some drugs have been reported to induce the type-1 ECG and/or (fatal) arrhythmias in Brugada syndrome patients. Patients with Brugada syndrome can prevent arrhythmias by avoiding these drugs or using them only in controlled conditions.[18] Those with risk factors for coronary artery disease may require an angiogram before ICD implantation.
See also
References
- ↑ Nademanee K; Veerakul G; Nimmannit S; et al. (1997). "Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men". Circulation. 96 (8): 2595–2600. doi:10.1161/01.cir.96.8.2595. PMID 9355899.
- ↑ Vatta M; Dumaine R; Varghese G; et al. (February 2002). "Genetic and biophysical basis of sudden unexplained nocturnal death syndrome (SUNDS), a disease allelic to Brugada syndrome". Hum. Mol. Genet. 11 (3): 337–45. doi:10.1093/hmg/11.3.337. PMID 11823453.
- ↑ Brugada J, Brugada P, Brugada R (July 1999). "The syndrome of right bundle branch block ST segment elevation in V1 to V3 and sudden death--the Brugada syndrome". Europace. 1 (3): 156–66. doi:10.1053/eupc.1999.0033. PMID 11225790.
- ↑ Martini B; Nava A; Thiene G; et al. (December 1989). "Ventricular fibrillation without apparent heart disease: description of six cases". Am. Heart J. 118 (6): 1203–9. doi:10.1016/0002-8703(89)90011-2. PMID 2589161.
- ↑ Brugada P, Brugada J (November 1992). "Right bundle branch block, persistent ST segment elevation and sudden cardiac death: a distinct clinical and electrocardiographic syndrome. A multicenter report". J. Am. Coll. Cardiol. 20 (6): 1391–6. doi:10.1016/0735-1097(92)90253-J. PMID 1309182.
- 1 2 Antzelevitch C; Pollevick GD; Cordeiro JM; et al. (2007). "Loss-of-Function Mutations in the Cardiac Calcium Channel Underlie a New Clinical Entity Characterized by ST-Segment Elevation, Short QT Intervals, and Sudden Cardiac Death". Circulation. 115 (4): 442–229. doi:10.1161/CIRCULATIONAHA.106.668392. PMC 1952683
 . PMID 17224476.
. PMID 17224476. - ↑ Delpon E; Cordeiro JM; Núñez L; et al. (2008). "Functional Effects of KCNE3 Mutation and its Role in the Development of Brugada Syndrome". Circulation Arrhythmia and Electrophysiology. 1 (3): 209–18. doi:10.1161/CIRCEP.107.748103. PMC 2585750
 . PMID 19122847.
. PMID 19122847. - ↑ Watanabe H; Koopmann TT; Le Scouarnec S; et al. (June 2008). "Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans". J. Clin. Invest. 118 (6): 2260–8. doi:10.1172/JCI33891. PMC 2373423
 . PMID 18464934.
. PMID 18464934. - 1 2 3 Bezzina, Connie R; Barc, Julien; Mizusawa, Yuka; Remme, Carol Ann; Gourraud, Jean-Baptiste; Simonet, Floriane; Verkerk, Arie O; Schwartz, Peter J; Crotti, Lia; Dagradi, Federica; Guicheney, Pascale; Fressart, Véronique; Leenhardt, Antoine; Antzelevitch, Charles; Bartkowiak, Susan; Schulze-Bahr, Eric; Zumhagen, Sven; Behr, Elijah R; Bastiaenen, Rachel; Tfelt-Hansen, Jacob; Olesen, Morten Salling; Kääb, Stefan; Beckmann, Britt M; Weeke, Peter; Watanabe, Hiroshi; Endo, Naoto; Minamino, Tohru; Horie, Minoru; Ohno, Seiko; Hasegawa, Kanae; Makita, Naomasa; Nogami, Akihiko; Shimizu, Wataru; Aiba, Takeshi; Froguel, Philippe; Balkau, Beverley; Lantieri, Olivier; Torchio, Margherita; Wiese, Cornelia; Weber, David; Wolswinkel, Rianne; Coronel, Ruben; Boukens, Bas J; Bézieau, Stéphane; Charpentier, Eric; Chatel, Stéphanie; Despres, Aurore; Gros, Françoise; Kyndt, Florence; Lecointe, Simon; Lindenbaum, Pierre; Portero, Vincent; Violleau, Jade; Gessler, Manfred; Tan, Hanno L; Roden, Dan M; Christoffels, Vincent M; Marec, Hervé Le; Wilde, Arthur A; Probst, Vincent; Schott, Jean-Jacques; Dina, Christian; Redon, Richard (2013). "Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death". Nature Genetics. 45: 1044–9. doi:10.1038/ng.2712. ISSN 1061-4036. PMC 3869788
 . PMID 23872634.
. PMID 23872634. - ↑ Genet Med. 2015 Apr 23. doi: 10.1038/gim.2015.35. Brugada syndrome: clinical and genetic findings. Sarquella-Brugada G1, Campuzano O2, Arbelo E3, Brugada J4, Brugada R5.
- 1 2 Hedley PL; Jørgensen P; Schlamowitz S; Moolman-Smook, Johanna; et al. (2009). "Brugada syndrome". Human Mutation. 30 (9): 1256–66. doi:10.1002/humu.21066. PMID 19606473.
- ↑ Antzelevitch C (2007). "Genetic basis of Brugada syndrome". Heart Rhythm. 4 (6): 756–7. doi:10.1016/j.hrthm.2007.03.015. PMC 1989771
 . PMID 17556198.
. PMID 17556198. - ↑ Arrhythmogenic Disorders of Genetic Origin Brugada Syndrome. Mizusawa, Y and Arthur A.M. Wilde, AM, Circulation: Arrhythmia and Electrophysiology. 2012; 5: 606-616 doi: 10.1161/CIRCEP.111.964577
- ↑ "Brugada syndrome Symptoms - Mayo Clinic". www.mayoclinic.org. Retrieved 2015-11-09.
- ↑ Antzelevitch C; Brugada P; Borggrefe M; et al. (2005). "Brugada Syndrome. Report of the second consensus conference". Heart Rhythm. 2 (4): 429–440. doi:10.1016/j.hrthm.2005.01.005. PMID 15898165.
- ↑ Kalavakunta, Jagadeesh K.; Bantu, Vishwaroop; Tokala, Hemasri; Kodenchery, Mihas (2010-01-01). "Sudden cause of cardiac death-be aware of me: a case report and short review on brugada syndrome". Case Reports in Medicine. 2010: 823490. doi:10.1155/2010/823490. ISSN 1687-9635. PMC 3014853
 . PMID 21209740.
. PMID 21209740. - ↑ Belhassen B, Glick A, Viskin S (2004). "Efficacy of quinidine in high-risk patients with Brugada syndrome". Circulation. 110 (13): 1731–7. doi:10.1161/01.CIR.0000143159.30585.90. PMID 15381640.
- ↑ Postema, PG; Wolpert, C; Amin, AS; Probst, V; Borggrefe, M; Roden, DM; Priori, SG; Tan, HL; Hiraoka, M; Brugada, J; Wilde, AA (September 2009). "Drugs and Brugada syndrome patients: review of the literature, recommendations and an up-to-date website (www.brugadadrugs.org)". Heart Rhythm. 6 (9): 1335–41. doi:10.1016/j.hrthm.2009.07.002. PMC 2779019
 . PMID 19716089.
. PMID 19716089.
External links
- BrugadaDrugs.org, maintained by Brugada specialists, contains a list of drugs to avoid in patients with the Brugada syndrome
- GeneReviews: Brugada syndrome
- Behr: https://web.archive.org/web/20021220063450/http://www.c-r-y.org.uk:80/long_qt_syndrome.htm
- The Ramon Brugada Senior Foundation
- la Sindrome di Brugada