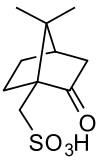
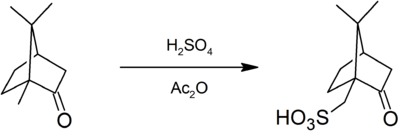Camphorsulfonic acid
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(7,7-dimethyl-2-oxobicyclo[2.2.1]heptan-1-yl)methanesulfonic acid | |
| Other names
Reychler's acid; 2-Oxobornane-10-sulfonic acid | |
| Identifiers | |
| 5872-08-2 35963-20-3 (1R) 3144-16-9 | |
| 3D model (Jmol) | Interactive image Interactive image |
| 2216194 | |
| ChEBI | CHEBI:55379 |
| ChemSpider | 17438 116050 (1R) 189449 (1S) 2318313 (4S) |
| ECHA InfoCard | 100.025.024 |
| EC Number | 227-527-0 |
| MeSH | 10-Camphorsulfonic+acid |
| PubChem | 18462 131278 (1R) 218580 (1S) 43833349 (4R) 3057042 (4S) |
| UNII | 9TLZ01S15L |
| UN number | 1759 |
| |
| |
| Properties | |
| C10H16O4S | |
| Molar mass | 232.29 g·mol−1 |
| Melting point | 195 °C (decomposes) |
| Acidity (pKa) | 1.2 |
| Hazards | |
| Safety data sheet | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Camphorsulfonic acid, sometimes abbreviated CSA or 10-CSA is an organosulfur compound. Like typical sulfonic acids, it is a relatively strong acid that is a colorless solid at room temperature and is soluble in water and a wide variety of organic substances.
This compound is commercially available. It can be prepared by sulfonation of camphor with sulfuric acid and acetic anhydride:[1]
In organic synthesis, CSA and its derivatives can be used as resolving agents for chiral amines and other cations.[2][3] For example, 3-bromocamphor-8-sulfonic acid was used in the synthesis of enantiopure devazepide.[4]
- Trimetaphan camsilate (trimethaphan camsylate) is another good example.
References
- ↑ Paul D. Bartlett and L. H. Knox (1973). "D,L-10-Camphorsulfonic acid (Reychler's Acid)". Org. Synth.; Coll. Vol., 5, p. 194
- ↑ D. Clark, Robin; R. Kern, John; J. Kurz, Lilia; T. Nelson, Janis (1990). "Preparation of Enatiomerically Pure Decahydro-6H-isoquino[2,1-g][1,6]naphthyridines Utilizing the Openshaw-Whittaker Hexahydrobenzo[a]quinolizinone Resolution". Heterocycles. 31 (2): 353. doi:10.3987/COM-89-5250.
- ↑ André B. Charette "3-Bromocamphor-8-sulfonic Acid" Encyclopedia of Reagents for Organic Synthesis 2001, John Wiley & Sons. doi:10.1002/047084289X.rb283
- ↑ Reider, Paul J.; Davis, Paul; Hughes, David L.; Grabowski, Edward J. J. (1987). "Crystallization-induced asymmetric transformation: Stereospecific synthesis of a potent peripheral CCK antagonist". J. Org. Chem. 52 (5): 955–957. doi:10.1021/jo00381a052.
This article is issued from Wikipedia - version of the 6/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.