Diastolic heart failure
| Diastolic dysfunction | |
|---|---|
| Classification and external resources | |
| ICD-9-CM | 428.3 |
Diastolic heart failure and diastolic dysfunction refer to the decline in performance of one (usually the left ventricle) or both (left and right) ventricles during diastole. Diastole is the cardiac cycle phase during which the heart is relaxing and filling with incoming blood that is being returned from the body through the inferior (IVC) and superior (SVC) venae cavae to the right atrium and from lungs through pulmonary veins to the left atrium. In diastolic failure, if the patient has symptoms, there is a pathologic cause inducing them. Diastolic dysfunction can be found when doing a Doppler echocardiography in an apparently healthy patient, mainly in an elderly person.[1]
Causes
Any condition or process that leads to stiffening of the left ventricle can lead to diastolic dysfunction. Causes of left ventricular stiffening include:
- A long-standing hypertension where, as a result of left ventricular muscle hypertrophy caused by the high pressure, the left ventricle has become stiff.
- Aortic stenosis of any cause where the ventricular muscle becomes hypertrophied, and thence stiff, as a result of the increased pressure load placed on it by the stenosis.
- Diabetes
- Age – elderly patients mainly if they have hypertension.
Causes of isolated right ventricular diastolic failure are uncommon. These causes include:
- Constrictive pericarditis
- Restrictive cardiomyopathy, which includes Amyloidosis (most common restrictive), Sarcoidosis and fibrosis.
Pathophysiology
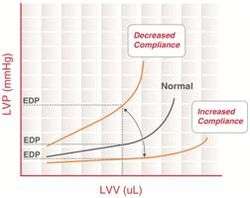
Diastolic failure appears when the ventricle can't be filled properly because it can't relax or because its wall is thick or rigid. This situation presents usually a concentric hypertrophy. In contrast, systolic heart failure has usually an eccentric hypertrophy.[2]
Diastolic failure is characterized by an elevated diastolic pressure in the left ventricle, despite an essentially normal/physiologic end diastolic volume (EDV). Histological evidence supporting diastolic dysfunction demonstrates ventricular hypertrophy, increased interstitial collagen deposition and infiltration of the myocardium. These influences collectively lead to a decrease in distensibility and elasticity (ability to stretch) of the myocardium. As a consequence, cardiac output becomes diminished. When the left ventricular diastolic pressure is elevated, venous pressure in lungs must also become elevated too: left ventricular stiffness makes it more difficult for blood to enter it from the left atrium. As a result, pressure rises in the atrium and is transmitted back to the pulmonary venous system, thereby increasing its hydrostatic pressure and promoting pulmonary edema.[1]
It may be misguided to classify the volume-overloaded heart as having diastolic dysfunction if it is behaving in a stiff and non-compliant manner. The term diastolic dysfunction should not be applied to the dilated heart. Dilated ("remodeled") hearts have increased volume relative to the amount of diastolic pressure, and therefore have increased (not decreased) distensibility. The term diastolic dysfunction is sometimes erroneously applied in this circumstance, when increased fluid volume retention causes the heart to be over-filled (High output cardiac failure).[1]
Although the term diastolic heart failure is often used when there are signs and symptoms of heart failure with normal left ventricular systolic function, this is not always appropriate. Diastolic function is determined by the relative end diastolic volume in relation to end diastolic pressure, and is therefore independent of left ventricular systolic function. A leftward shift of the end-diastolic pressure-volume relationship (i.e. decreased left ventricular distensibility) can occur both in those with normal and those with decreased left ventricular systolic function. Likewise, heart failure may occur in those with dilated left ventricular and normal systolic function. This is often seen in valvular heart disease and high-output heart failure. Neither of these situations constitutes a diastolic heart failure.[1]
Physiology

In diastolic heart failure, "the volume of blood contained in the ventricles during diastole is lower than it should be, and the pressure of the blood within the chambers is elevated."[3]
Diastole
During diastole, the ventricular pressure falls from the peak reached at the end of systole. When this pressure falls below the atrial pressure, atrio-ventricular valves open (mitral valve at left side and tricuspid valve at right side) and the blood passes from the atria into the ventricles. First, ventricles are filled by a pressure gradient but near the end, atria contract (atrial kick) and force more blood to pass into ventricles. Atrial contraction is responsible for around 20% of the total filling blood volume. (In atrial fibrillation, this additional 20% filling volume is lost and the patient may experience systolic heart failure symptoms).[4] Complete left ventricular filling is essential to maintain maximum cardiac output. Left ventricular filling is dependent upon ventricular relaxation and compliance, mitral valve area, atrio-ventricular gradient, atrial contraction and end-systolic volume. Diastole has four phases: isovolumetric relaxation, rapid filling, diastasis and atrial contraction. All of these phases can be evaluated by Doppler echocardiography.[1]
Diagnosis
Diastolic dysfunction must be differentiated from diastolic heart failure. Diastolic dysfunction can be found in elderly and apparently quite healthy patients. If diastolic dysfunction describes an abnormal mechanical property, diastolic heart failure describes a clinical syndrome. Mathematics describing the relationship between the ratio of Systole to Diastole in accepted terms of End Systolic Volume to End Diastolic Volume implies many mathematical solutions to forward and backward heart failure.
Criteria for diagnosis of diastolic dysfunction or diastolic heart failure remain imprecise. This has made it difficult to conduct valid clinical trials of treatments for diastolic heart failure. The problem is compounded by the fact that systolic and diastolic heart failure commonly coexist when patients present with many ischemic and nonischemic etiologies of heart failure. Narrowly defined, diastolic failure has often been defined as "heart failure with normal systolic function" (i.e. left ventricular ejection fraction of 60% or more). Chagasic heart disease may represent an optimal academic model of diastolic heart failure that spares systolic function.
A patient is said to have diastolic dysfunction if he has signs and symptoms of heart failure but the left ventricular ejection fraction is normal. A second approach is to use an elevated BNP level in the presence of normal ejection fraction to diagnose diastolic heart failure. Concordance of both volumetric and biochemical measurements and markers lends to even stronger terminology regarding scientific/mathematical expression of diastolic heart failure. These are both probably too broad a definition for diastolic heart failure, and this group of patients is more precisely described as having heart failure with normal systolic function. Echocardiography can be used to diagnose diastolic dysfunction but is a limited modality unless it is supplemented by stress imaging. MUGA imaging is an earlier mathematical attempt to distinguish systolic from diastolic heart failure.
No one single echocardiographic parameter can confirm a diagnosis of diastolic heart failure. Multiple echocardiographic parameters have been proposed as sufficiently sensitive and specific, including mitral inflow velocity patterns, pulmonary vein flow patterns, E:A reversal, tissue Doppler measurements, and M-mode echo measurements (i.e. of left atrial size). Algorithms have also been developed which combine multiple echocardiographic parameters to diagnose diastolic heart failure.
There are four basic Echocardiographic patterns of diastolic heart failure, which are graded I to IV:
- The mildest form is called an "abnormal relaxation pattern", or grade I diastolic dysfunction. On the mitral inflow Doppler echocardiogram, there is reversal of the normal E/A ratio. This pattern may develop normally with age in some patients, and many grade I patients will not have any clinical signs or symptoms of heart failure.
- Grade II diastolic dysfunction is called "pseudonormal filling dynamics". This is considered moderate diastolic dysfunction and is associated with elevated left atrial filling pressures. These patients more commonly have symptoms of heart failure, and many have left atrial enlargement due to the elevated pressures in the left heart.
Grade III and IV diastolic dysfunction are called "restrictive filling dynamics". These are both severe forms of diastolic dysfunction, and patients tend to have advanced heart failure symptoms:
- Class III diastolic dysfunction patients will demonstrate reversal of their diastolic abnormalities on echocardiogram when they perform the Valsalva maneuver. This is referred to as "reversible restrictive diastolic dysfunction".
- Class IV diastolic dysfunction patients will not demonstrate reversibility of their echocardiogram abnormalities, and are therefore said to suffer from "fixed restrictive diastolic dysfunction".
The presence of either class III and IV diastolic dysfunction is associated with a significantly worse prognosis. These patients will have left atrial enlargement, and many will have a reduced left ventricular ejection fraction that indicates a combination of systolic and diastolic dysfunction.
Imaged volumetric definition of systolic heart performance is commonly accepted as ejection fraction. Volumetric definition of the heart in systole was first described by Adolph Fick as cardiac output. Fick may be readily and inexpensively inverted to cardiac input and injection fraction to mathematically describe diastole. Decline of injection fraction paired with decline of E/A ratio seems a stronger argument in support of a mathematical definition of diastolic heart failure.
Another parameter to assess diastolic function is the E/E' ratio, which is the ratio of mitral peak velocity of early filling (E) to early diastolic mitral annular velocity (E'). Diastolic dysfunction is assumed when the E/E' ratio exceed 15.[5]
Treatment
Generally, diastolic dysfunction is a chronic process. When this chronic condition is well tolerated by an individual, no specific treatment may be indicated. Rather, therapy should be directed at the root cause of the stiff left ventricle, with potential causes and aggravating factors like high blood pressure and diabetes treated appropriately. Conversely (as noted above), diastolic dysfunction tends to be better tolerated if the atrium is able to pump blood into the ventricles in a coordinated fashion. This does not occur in atrial fibrillation (AF), where there is no coordinated atrial activity and the left ventricle loses around 20% of its output. However, in chronic AF and in geriatric patients, AF is better tolerated and the cardiologist must choose between a stable AF at a lower rate and the risk of having an intermittent AF if he pretends to treat AF aggressively with all the thrombo-embolic risk it implies. In the same light, and also as noted above, if the atrial fibrillation persists and is resulting in a rapid heart rate, treatment must be given to slow down that rate. Usually digoxin maintains a stable rhythm. The use of a self-expanding device that attaches to the external surface of the left ventricle has been suggested, yet still awaits FDA approval. When the heart muscle squeezes, energy is loaded into the device, which absorbs the energy and releases it to the left ventricle in the diastolic phase. This helps retain muscle elasticity.[6]
The role of specific treatments for diastolic dysfunction per se is as yet unclear. Diuretics can be useful, if these patients develop significant congestion, but patients must be monitored because they frequently develop hypotension.[7]
Beta-blockers are the first-line therapy as they induce bradycardia and give time for ventricles to fill. There is some evidence that calcium channel blocker drugs may be of benefit in reducing ventricular stiffness in some cases (verapamil has the benefit lowering the heart rate). Likewise, treatment with angiotensin converting enzyme inhibitors, such as enalapril, ramipril, and many others, may be of benefit due to their effect on preventing ventricular remodeling but under control to avoid hypotension.[7]
Prognosis
Until recently, it was generally assumed that the prognosis for individuals with diastolic dysfunction and associated intermittent pulmonary edema was better than those with systolic dysfunction. In fact, in two studies appearing in the New England Journal of Medicine in 2006, evidence was presented to suggest that the prognosis in diastolic dysfunction is the same as that in systolic dysfunction.[8][9]
Bibliography
- Estafanous, F.G. (2001). Cardiac anesthesia 2 Ed: Principles and clinical practice (2 ed.). Lippincott Williams & Wilkins. ISBN 978-0781721950.
- O'Rouke, R.A., Fuster, V. (2001). Hurst's The Heart (10 (International edition) ed.). McGraw-Hill. pp. 658–60. ISBN 0-07-116296-8.
See also
References
- 1 2 3 4 5 Hurst 2001, pp. 658–60.
- ↑ Eric J. Topol; Robert M. Califf (2007). Textbook of cardiovascular medicine. Lippincott Williams & Wilkins. pp. 420–. ISBN 978-0-7817-7012-5. Retrieved 16 November 2010.
- ↑ Crowley, Leonard V. (2013). An Introduction to Human Disease: Pathology and Pathophysiology Correlations. Jones & Bartlett Publishers. p. 323. ISBN 9781449632403. Retrieved 16 August 2014.
In this condition, called diastolic heart failure, the volume of blood contained in the ventricles during diastole is lower than it should be, and the pressure of the blood within the chambers is elevated.
- ↑ Estafanous 2001.
- ↑ Germing, A.; Gotzmann, M.; Schikowski, T.; Vierkötter, A.; Ranft, U.; Krämer, U.; Mügge, A. (2011). "High frequency of diastolic dysfunction in a population-based cohort of elderly women - but poor association with the symptom dyspnea". BMC Geriatrics. 11: 71. doi:10.1186/1471-2318-11-71. PMC 3219735
 . PMID 22047619.
. PMID 22047619. - ↑
- 1 2 Hurst 2001, p. 709.
- ↑ Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM (July 2006). "Trends in prevalence and outcome of heart failure with preserved ejection fraction". N. Engl. J. Med. 355 (3): 251–59. doi:10.1056/NEJMoa052256. PMID 16855265.
- ↑ Bhatia RS, Tu JV, Lee DS, et al. (July 2006). "Outcome of heart failure with preserved ejection fraction in a population-based study". N. Engl. J. Med. 355 (3): 260–69. doi:10.1056/NEJMoa051530. PMID 16855266.