Diloxanide
 | |
| Clinical data | |
|---|---|
| Trade names | Furamide |
| AHFS/Drugs.com | Micromedex Detailed Consumer Information |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | P01AC01 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 90% (diloxanide) |
| Metabolism | Hydrolyzed to furoic acid and diloxanide, which undergoes extensive glucuronidation |
| Biological half-life | 3 hours |
| Excretion | Renal (90%), fecal (10%) |
| Identifiers | |
| |
| CAS Number |
3736-81-0 |
| PubChem (CID) | 19529 |
| DrugBank |
DB08792 |
| ChemSpider |
18400 |
| KEGG |
D02480 |
| ChEMBL |
CHEMBL1334860 |
| Chemical and physical data | |
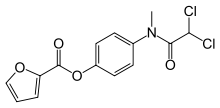
| Formula | C14H11Cl2NO4 |
| Molar mass | 328.147 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Diloxanide furoate is a medication used to treat infection with amoebas. It works only in the digestive tract. Diloxanide is considered second line treatment after paromomycin when no symptoms are present but the person is passing cysts, in places where infections are not common.[1] For people who are symptomatic, it is used after treatment with ambecides that can penetrate tissue, like metronidazole or tinidazole.[1] Paromomycin is considered first line treatment and diloxanide is considered second line treatment for this use as well.[1]
Pregnant women should not take it, as it may harm the baby.[2] Otherwise it is very safe with the main side effect being flatulence.[3] It is a lumenal amebicide.[1]
Diloxanide furoate is on the World Health Organization's List of Essential Medicines, of the most important medication needed in a basic health system.[4] The drug was discovered by Boots UK in 1956 and introduced as Furamide. It was not available in much of the developed world as of 2012.[2]
Medical uses
Dloxanide furoate works only in the digestive tract and is a lumenal amebicide.[1][3] It is considered second line treatment for infection with amoebas when no symptoms are present but the person is passing cysts, in places where infections are not common.[1][5] Paromomycin is considered the first line treatment for these cases.
For people who are symptomatic, it is used after treatment with ambecides that can penetrate tissue, like metronidazole or tinidazole. Diloxanide is considered second-line, while paromomycin is considered first line for this use as well.[1][6]
Contraindication
Diloxanide furoate is contraindicated in pregnant women, women who are breast feeding, and in children <2 years of age.[2]
Adverse effects
Side effects include flatulence, itchiness, and hives. In general, the use of diloxanide is well tolerated with minimal toxicity. Although there is no clear risk of harm when used during pregnancy, diloxanide should be avoided in the first trimester if possible.[3]
Pharmacology
Diloxanide furoate destroys trophozoites of E. Histolica and prevents ameobic cyst formation.[7] The exact mechanism of diloxanide is unknown.[8] Diloxanide is structurally related to chloramphenicol and may act in a similar fashion by blocking protein synthesis.[2]
The prodrug, diloxanide furoate, is metabolized in the gastrointestinal tract to release the active drug, diloxanide.[8]
90% of each dose is excreted in the urine and the other 10% is excreted in the feces.[8]
Society and culture
Diloxanide furoate is on the World Health Organization's List of Essential Medicines, of the most important medication needed in a basic health system.[4]
The drug was discovered by Boots UK in 1956 and introduced as Furamide; it was not available in much of the developed world as of 2012.[2] As of 2014, in the case of emergencies, diloxanide could be acquired from the Parasitic Disease Drug Service of the Centers for Disease Control and Prevention in the US.[9]
References
- 1 2 3 4 5 6 7 Farthing, Michael JG (August 2006). "Treatment options for the eradication of intestinal protozoa". Nature Clinical Practice Gastroenterology & Hepatology. 3 (8): 436–445. doi:10.1038/ncpgasthep0557. PMID 16883348.
- 1 2 3 4 5 Griffin, Paul M (2012). "Chapter 181: Diloxanide furoate". In Grayson, M. Lindsay. Kucers' the use of antibiotics a clinical review of antibacterial, antifungal, antiparasitic and antiviral drugs (6th ed. ed.). Boca Raton, FL: CRC Press. p. 2121. ISBN 9781444147520.
- 1 2 3 "Protozoa: Amoebiasis and giardiasis: Diloxanide". WHO Model Prescribing Information: Drugs Used in Parasitic Diseases (2nd ed.). WHO. 1995. ISBN 92 4 140104 4.
- 1 2 "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ McAuley JB, Herwaldt BL, Stokes SL, et al. (1992). "Diloxanide furoate for treating asymptomatic Entamoeba histolytica cyst passers: 14 years' experience in the United States". Clin. Infect. Dis. 15 (3): 464–8. doi:10.1093/clind/15.3.464. PMID 1520794.
- ↑ Arcangelo, Virginia Poole (2006). Pharmacotherapeutics For Advanced Practice: A Practical Approach. Lippincott Williams and Wilkins. p. 441. ISBN 978-0-7817-5784-3.
- ↑ Gupta, Y. K.; Gupta, Madhur; Aneja, S.; Kohli, K. (January 2004). "Current drug therapy of protozoal diarrhoea". The Indian Journal of Pediatrics. 71 (1): 55–58. doi:10.1007/BF02725657. PMID 14979387.
- 1 2 3 "Diloxanide 500 mg Tablets - Summary of Product Characteristics". UK Electronic Medicines Compendium. March 31, 2015. Retrieved 11 November 2016.
- ↑ Thomas, Stephen J., ed. (2014). Walter Reed Army Institute of Research Tropical Medicine Course Laboratory Manual (PDF) (7th ed.). U.S. Department of the Army. p. 34.