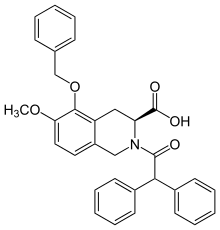
EMA401
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 33% |
| Protein binding | Angiotensin II Subtype 2 Receptor |
| Biological half-life | 6-12 hr |
| Identifiers | |
| |
| CAS Number | 1316755-17-5 |
| PubChem (CID) | 9937291 |
| ChemSpider | 8068121 |
| ChEBI | CHEBI:150942 |
| ChEMBL | CHEMBL34124 |
| Chemical and physical data | |
| Formula | C32H29NO5 |
| Molar mass | 507.58 g/mol |
| 3D model (Jmol) | Interactive image |
| Specific rotation | + |
| Density | 1.256±0.06 g/cm3 |
| Boiling point | 745.3 ± 60.0 °C (1,373.5 ± 108.0 °F) |
| Solubility in water | 14 mg/mL (20 °C) |
| |
| |
EMA401 is a new drug under development for the treatment of peripheral neuropathic pain. It was recently established as a potential drug option for patients suffering pain caused by postherpetic neuralgia.[1] It may also be useful for treating various types of chronic neuropathic pains caused by lesions and other diseases affecting the somatosensory system in addition to postherpetic neuralgia.[2] EMA401 has been implicated in alleviating pains associated with a host of neural abnormalities, ranging from excessive nerve sprouting due to damaged nerve caused by shingles, diabetes, osteoarthritis, HIV and chemotherapy.[3][4][5][6] EMA401 is a competitive antagonist of angiotensin II type 2 receptor (AT2R) being developed by the Australian biotechnology company Spinifex Pharmaceuticals. It is the S-enantiomer form of the racemate drug EMA400.[7] Analgesic treatments currently available for pain disorders are unsuited for severe chronic neuropathic pains. EMA401 is more effective and has virtually no central nervous system side effects in comparison to current drugs for pain relief, such as Neurotin and Lyrica.[8] Pain pathways of the other functional systems have major molecular and mechanistic differences compared to pain pathways of the peripheral nervous system. EMA401 target proteins, angiotensin II type 2 receptors, are extremely important for nociception within the peripheral nervous system and less so for nociception within other functional systems.[9] EMA401 is the first drug on the market that targets angiotensin II type 2 receptors (AT2R) with high affinity but has a low affinity for angiotensin II type 1 receptors. Angiotensin II receptor antagonists that have existed in the pharmaceutical market have had higher affinities for angiotensin II type 1 receptors (AT1R) over type 2 receptors. AT1R are not involved with nociception. AT1R antagonists have been mostly used for the treatment of hypertension.
History of drug development
Angiotensin II is an octapeptide hormone central to the renin-angiotensin system.It regulates blood pressure control, water fluid homeostasis, and neuronal excitability.[10][11][12][13] Receptor agonists and antagonist of angiotensin II receptors that target various parts of the complicated renin-angiotensin system were developed to increase knowledge of the renin-angiotensin system and aid the development of antihypertensive drug candidates.[14][15] These investigations led to the discovery of two subtypes of membrane bound G protein-coupled angiotensin receptors within the renin-angiotensin system with vastly different functions: angiotensin II type 1 receptors (AT1R) and angiotensin II type 2 receptors (AT2R). AT1R is the receptor subtype that was found to be mainly responsible for blood pressure, water fluid regulation, and other classical known physiological actions of angiotensin II on the renin-angiotensin system.[16] Drugs that antagonize AT1R have become used as common treatments for hypertension and congestive heart failure. Functions of AT2R were unknown in comparison.[17]
Development of selective AT2R agonists and antagonists aided the discovery of AT2Rs' functional roles within the nervous system.[18] 4,5,6,7-tetrahydro-1H-imidazo[4,5-]pyridine derivatives were the first compounds described to have antagonizing properties at the AT2 receptors.[19] Inventors at Warner-Lambert Company LLC substituted 1,2,3,4-tetrahydroisoquinoline with various functional groups to create numerous compounds of formula I that had improved affinity for AT2R.[20] EMA400, the racemate drug to EMA401, was originally Compound 6, one of the 48 compounds of formula I. Compound 6 had higher binding affinity for AT2R, compared to the other 48 compounds, with an IC50 value of 2.8 nM in a rabbit uterus binding assay. It was also inactive in the AT1R binding assay at concentrations of 10-5 M, suggesting high selectivity for AT2R. Example 47, the S-enantiomer of Compound 6 and later named EMA401, was at the time an unanalyzed compound of formula I.[21] Compound 6, under the name PD-126,055, and five other AT2R antagonists (PD-123,319, L-159,686, PD-121,981, L-161,638 and L-163,579) were reported to have previously unknown analgesic properties by investigators from the University of Queensland.[22] Greater understanding of the pathobiology of neuropathic pain had been and still is of high demand, as is better neuropathic pain relief medication. By 2020, the neuropathic pain treatment market is projected to reach $3.6 billion USD.[23] The report on those six drugs was the first to clearly elucidate the role of angiotensin II type 2 receptors in neuropathic pain and implicated AT2R antagonists as potential drugs for the treatment of neuropathic pain.
Angiotensin II AT2 receptors are produced locally in nociceptive tissues.[24] Nociceptive neurons express only AT2R, not AT1R. PD-123,319, commonly used in early angiotensin II research for its moderate inhibition of AT2R, blocks angiotensin II induced neuronal excitability in cultured cells of neuronal origins and also in cultured dorsal root ganglion neurons from adult rats.[25] The family of small molecule inhibitors of angiotensin II activity on AT2R, EMA200 (PD-123,319), EMA300 (PD-121,981), and EMA400 (PD-126,055), relieved hindpaw sensitivity in a rat model of neuropathic pain in a recent study conducted at University of Queensland. Relief of hindpaw hypersensitivity was dose dependent. EMA400 had the highest antineuropathic potency, 60-fold more potent than EMA300 and 250-fold more potent than EMA200. EMA400 had an IC50 of 75.2 nM for rat AT2R and an IC50 of 2.9x106 nM for rat AT1R. EMA400, a racemate, was separated into EMA401, the S-enantiomer of EMA400, and EMA402, the R-enantiomer of EMA400. EMA401 had an IC50 of 39 nM for human AT2R, compared to EMA402 with an IC50 of 1,100 nM for human AT2R. EMA401 was selected as the candidate drug for neuropathic pain treatment for clinical development.
Animal studies, five Phase I clinical trials, unpublished data at Spinifex Pharmaceuticals, implicated a dose range of 10–50 mg per day of EMA401 to be effective on human beings. Phase I clinical trial also indicated EMA401 dose of up to 400 mg to be safe.[26] Spinifex Pharmaceuticals reported the results of a phase 2 clinical trial results in which 183 patients with postherpetic neuralgia received either oral EMA401 or placebo for 28 days.[27] Those assigned to the active condition receiving EMA401 reported significantly less postherpetic neuralgia, a chronic neuropathic pain, compared to those in the placebo condition. There was no evidence of any serious side effects caused by EMA401. Spinifex Pharmaceuticals plans to proceed with larger phase II clinical trial to test higher doses for longer periods of time. A nonrandomized Phase 2 study of EMA401 for the treatment of pain in patients with chemotherapy-induced peripheral neuropathy was approved and is currently underway.
Mechanism of action
Nociceptive pain is immediate pain felt as a result of injury inflicted upon tissue. Neuropathic pain, in contrast, is long-lasting pain that develops when the peripheral and the central nervous system are sensitized following injury to these systems. Neuropathic pain may be continuous pain, spontaneous, or pain caused by hypersensitivity to non-nociceptive levels of stimulus.[28] Angiotensin II (AngII) is an octapeptide that regulates blood pressure, controls water fluid balance, and pain perception. It activates two membrane bound G protein-coupled receptors: angiotensin II type 1 receptors (AT1R) and angiotensin II type 2 receptors (AT2R).[29] AgnII co-localization with substance P and calcitonin gene-related peptide implies its role in nociception.[30] AT2R, the receptor mainly responsible for AngII's effect on the pain mechanisms within the nervous system, is expressed in the dorsal root ganglion (DRG), as well as human trigeminal ganglia.[31] These receptors are locally produced inside DRG neurons, implying the presence of an intrinsic angiotensinergic system. Injured nerves and painful neuromas show higher AT2R expression compared to normal undamaged nerves.[32] AngII contributes to neuropathic pain by potentiating neuronal pathways that signal pain when AngII activates AT2R expressed on pain detecting neurons. AngII potentiates neuronal pathways by increasing neurite length and density in DRG neurons.[33] AngII potentiates neuronal pathways by sensitizing nociceptors as well.[34] Nociceptor sensitization suggests a decreased thresholds for activation of nociceptors. AT2R activation increases kinase levels in DRG neurons, such as levels of PKA, PKC and MAPK,[35] via G protein-coupled receptor-activated second messenger pathways.[36] These phosphorylating enzymes sensitize major voltage-gated ion channels found on DRG neurons. Sensitization increases neuronal excitation following receptor channel activation. EMA401 antagonized human AT2R with a Ki of 5.4 nM, IC50 of 39 nM and a Hill coefficient of 1.3.[37] EMA401 alleviates pain and provides relief by blocking the AngII induced potentiation.
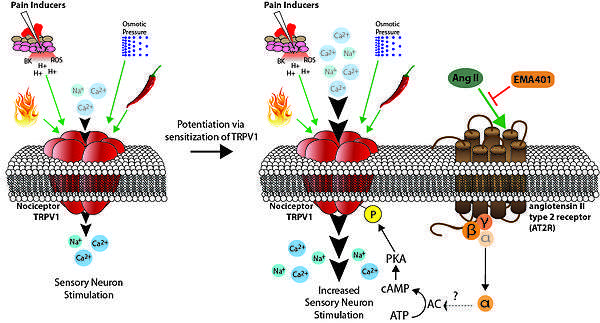
TRPV1 is an example a nociceptor that's sensitization is inhibited by EMA401. Clinical conditions of chronic pain shows up-regulated TRPV1 expression. The transient receptor potential cation channel subfamily V member 1 (TRPV1) is a ligand-gated non-selective cation channel found in sensory neurons that allows the sensation and perception of heat and other noxious stimuli. Opening of TRPV1 channels lead to the influx of Na2+ and Ca2+, causing the nociceptive neuron to depolarize. The direct phosphorylation of TRPV1 channel's Ser116 residue by cAMP-dependent protein kinase (PKA) sensitizes the TRPV1 channel.[38] cAMP signals PKA to phosphorylate TRPV1. Phosphorylated TRPV1 has a lower threshold for activation, causing potentiation of its neuronal pathways. Activation of the AT2R increases cAMP levels, resulting in higher numbers of phosphorylated TRPV1.[39] This causes behavioral hypersensitivity of TRPV1 to its stimuli. Increased AT2R activity causes hyperalgesia. AT2R antagonist EMA401 inhibits the downstream effects of AT2R activation, including an increase in cAMP. Fewer TRPV1 are phosphorylated when treated with EMA401, both reversing and/or preventing potentiation that causes hyperalgesia. AngII increases neuronal excitability to TRPV1 channel activation. EMA401 inhibits this increase in excitability in a dose dependent manner, producing analgesic effects.
Pharmacokinetics
EMA401 is the sodium-salt form of (S)-2-(diphenylacetyl)-l,2,3,4-tetrahydro-6-methoxy-5-(phenylmethoxy)- 3-isoquinoline carboxylic acid. EMA401 dose of up to 400 mg was tested in healthy male adults without any presentation of complications indicating safety issues.[40] EMA401 has been administered orally at a standardized dose of 100 mg twice daily. EMA401 reaches a maximum plasma concentration of 1000 ug/L one-hour after administration of 100 mg of EMA401 in both men and women, as observed in a phase II trial.[41] EMA401 has a t1/2 of 6 hours on day 1 of drug intake and a t1/2 of 12 hours on day 7 of drug intake. AUC averages around 1400 hr*ug/L at the given dose. Total plasma concentration of EMA401 is consistent over 28 days. A steady state of minimum drug plasma concentration is reached by the 8th day of being on the drug. EMA401 does not accumulate in the blood at presently administered doses. EMA401 does not cross the blood-brain barrier, therefore has little effect on the central nervous system.[42] Blood circulation delivers the drug to the dorsal root ganglion located on the dorsal root of the spine.
Adverse effects
No serious adverse effects have been determined that directly associates with EMA401.[43] Slightly higher frequency of complaints such as pharyngitis, headaches and allergic dermatitis are reported by individuals taking EMA401. However, pharyngitis was only reported in the placebo group of the phase 1 trial while no allergic dermatitis was reported in phase 1.[44] Headache frequency was higher in patients receiving EMA401 over placebo in both phase 1 and phase 2 clinical trials for EMA401.[45] The mechanism of why EMA401 may increases frequency of headaches is unknown. It is possible that a small amount of EMA401 is crossing the blood-brain barrier and affecting some functions of the CNS in the few patients that showed side-effects to EMA401. In consideration of the fact that the renin-angiotensin system regulates pressure within the cardiovascular system, patients taking EMA401 did not show any difference in vital signs or 12-lead ECG assessments compared to the patients taking placebo.
Synthesis of EMA401
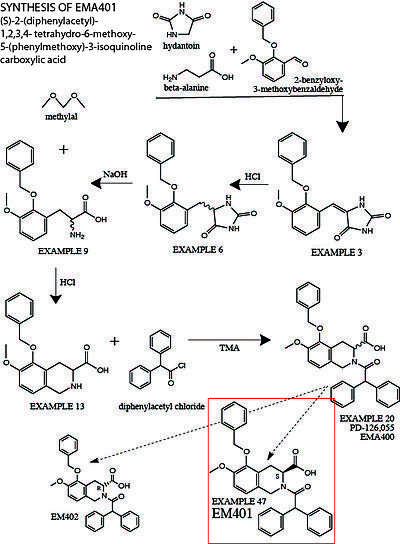
The following is a summary of the original synthesis of EMA401, described in detail in US5246943A.[46] More modified protocols have recently been established to synthesize EMA401 at a larger scale.[47] 2-benzyloxy-3-methoxybenzaldehyde, beta-alanine and hydantoin are stirred in glacial acetic acid for several hours to produce 5-[[3-Methoxy-2-(phenylmethoxy)phenyl]methylene]-2,4-imidazolidinedione (Example 3). Example 3 is treated with concentrated hydrochloric acid and heated to protonate the C-C double bond between the hydantoin group and the central benzene ring to create the racemate (RS)-5-[[3-Methoxy- 2-(phenylmethoxy)phenyl]methyl]-2,4-imidazolidinedione (Example 6). The hydantoin group is then hydrolyzed by heating Example 6 in sodium hydroxide solution to yield the racemic amino acid (RS)-3-Methoxy-2-(phenylmethoxy)phenylalanine (Example 9). Example 9 is then treated with methylal in a hydrochloric acid solution overnight to form (RS)-1,2,3,4-Tetrahydro-6-methoxy-5- phenylmethoxy-3-isoquinolinecarboxylic acid (Example 13). Example 13 is dissolved in a methylene chloride solution, treated with tetramethylammonium hydroxide and diphenylacetyl chloride to form (RS)-2-(Diphenylacetyl)-1,2,3,4-tetrahydro-6-methoxy-5-(phenylmethoxy)-3-isoquinolinecarboxylic acid (Example 20, aka. Compound 6, PD-126,055, EMA400).
In order to separate out the S-enantiomer of Example 20 from the racemate, Example 20 is dissolved in ethyl acetate and treated with chiral resolution reagent 1(−)-α-methylbenzylamine. Adding petroleum ether induces the crystallization of the less soluble diastereomeric salt, the S-enantiomer of Example 20 (Example 47). Deprotonated salt-crystals of the S-enantiomer Eample 47 are filtered, dissolved and protonated in methanol, and treated with 1% potassium bisulfate solution to precipitate out the free carboxylic acid Example 47. This chiral end product is (S)-(+)-2-(Diphenylacetyl)-1,2,3,4-tetrahydro-6-methoxy-5-(phenylmethoxy)-3-isoquinolinecarboxylic acid, the neutral form of the deprotonated sodium-salt that is EMA401.
References
- ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM (2013). "Protective arms of the renin-angiotensin-system in neurological disease". Clin Exp Pharmacol Physiol. 40 (8): 580–588. doi:10.1111/1440-1681.12137.
- ↑ WO patent 2006066361, SMITH MAREE THERESE; WYSE BRUCE DOUGLAS, "Methods of Treatment or Prophylaxis", published 2006-06-29
- ↑ WO patent 2011088504, MCCARTHY THOMAS DAVID; BAKER ANDREW RAINSFORD, "METHODS AND COMPOSITIONS FOR IMPROVED NERVE CONDUCTION VELOCITY", published 2006-06-29
- ↑ Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM (2013). "Protective arms of the renin-angiotensin-system in neurological disease". Clin Exp Pharmacol Physiol. 40 (8): 580–588. doi:10.1111/1440-1681.12137.
- ↑ Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P (2013). "Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons.". Eur J Pain. 17 (7): 1012–26. doi:10.1002/j.1532-2149.2012.00269.x.
- ↑ Maree T. Smith, P.; P. Bruce; D. Wyse; P. Stephen & R. Edwards (2013). "Small Molecule Angiotensin II Type 2 Receptor (AT2R) Antagonists as Novel Analgesics for Neuropathic Pain: Comparative Pharmacokinetics, Radioligand Binding, and Efficacy in Rats.". Pain Medicine. 14: 692–705. doi:10.1111/pme.12063.
- ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ WO patent 2006066361, SMITH MAREE THERESE; WYSE BRUCE DOUGLAS, "Methods of Treatment or Prophylaxis", published 2006-06-29
- ↑ Maree T. Smith, P. & P. Bruce D. Wyse, P. Stephen R. Edwards (2013). "Small Molecule Angiotensin II Type 2 Receptor (AT2R) Antagonists as Novel Analgesics for Neuropathic Pain: Comparative Pharmacokinetics, Radioligand Binding, and Efficacy in Rats.". Pain Medicine. 14: 692–705. doi:10.1111/pme.12063.
- ↑ Alterman M. (2010). "Development of selective non-peptide angiotensin II type 2 receptor agonists". J Renin Angiotensin Aldosterone Syst. 11 (1): 57–66. doi:10.1177/1470320309347790.
- ↑ US patent 4812462, BLANKLEY C JOHN; HODGES JOHN C, "4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid analogs having antihypertensive activity", published 2006-06-29
- ↑ US patent 5246943A, BLANKLEY CLIFTON J; HODGES JOHN C, "Substituted 1,2,3,4-Tetrahydroisoquinolines with Angiotensin II Receptor Antagonist Properties", published 1993-07-21
- ↑ US patent 4812462, BLANKLEY C JOHN; HODGES JOHN C, "4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid analogs having antihypertensive activity", published 2006-06-29
- ↑ US patent 5385894, DE LASZLO STEPHEN E; GLINKA TOMASZ W; GREENLEE WILLIAM J; CHAKRAVARTY PRASUN K; PATCHETT ARTHUR A, "Disubstituted 6-aminoquinazolinones", published 1997-01-31
- ↑ John H. Bauer, MD; Garry P. Reams, MD (1995). "The Angiotensin II Type 1 Receptor AntagonistsA New Class of Antihypertensive Drugs". Arch Intern Med. 155 (13): 1361–1368. doi:10.1001/archinte.1995.00430130027004.
- ↑ Steckelings UM, Kaschina E, Unger T (2005). "The AT2 receptor--a matter of love and hate". Peptides. 26 (8): 1401–9. doi:10.1016/j.peptides.2005.03.010. PMID 16042980.
- ↑ Alterman M. (2010). "Development of selective non-peptide angiotensin II type 2 receptor agonists". J Renin Angiotensin Aldosterone Syst. 11 (1): 57–66. doi:10.1177/1470320309347790.
- ↑ US patent 4812462, BLANKLEY C JOHN; HODGES JOHN C, "4,5,6,7-tetrahydro-1H-imidazo[4,5-c]pyridine-6-carboxylic acid analogs having antihypertensive activity", published 2006-06-29
- ↑ US patent 5246943A, BLANKLEY CLIFTON J; HODGES JOHN C, "Substituted 1,2,3,4-Tetrahydroisoquinolines with Angiotensin II Receptor Antagonist Properties", published 1993-07-21
- ↑ US patent 5246943A, BLANKLEY CLIFTON J; HODGES JOHN C, "Substituted 1,2,3,4-Tetrahydroisoquinolines with Angiotensin II Receptor Antagonist Properties", published 1993-07-21
- ↑ WO patent 2006066361, SMITH MAREE THERESE; WYSE BRUCE DOUGLAS, "Methods of Treatment or Prophylaxis", published 2006-06-29
- ↑ Nightingale, S (2012). "The neuropathic pain market". Nat Rev Drug Discov. 11 (2): 101–102. doi:10.1038/nrd3624.
- ↑ Steckelings UM, Kaschina E, Unger T (2005). "The AT2 receptor--a matter of love and hate". Peptides. 26 (8): 1401–9. doi:10.1016/j.peptides.2005.03.010. PMID 16042980.
- ↑ Maree T. Smith, P. & P. Bruce D. Wyse, P. Stephen R. Edwards (2013). "Small Molecule Angiotensin II Type 2 Receptor (AT2R) Antagonists as Novel Analgesics for Neuropathic Pain: Comparative Pharmacokinetics, Radioligand Binding, and Efficacy in Rats.". Pain Medicine. 14: 692–705. doi:10.1111/pme.12063.
- ↑ Spinifex Pharmaceuticals Pty Limited (2011). "CLINICAL STUDY PROTOCOL: Protocol No. EMA401-003". External link in
|title=(help) - ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ Basbaum AI, Bautista DM, Scherrer G, Julius D (2013). "Cellular and molecular mechanisms of pain". Cell. 139 (2): 267–284. doi:10.1016/j.cell.2009.09.028.
- ↑ Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P (2013). "Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons.". Eur J Pain. 17 (7): 1012–26. doi:10.1002/j.1532-2149.2012.00269.x.
- ↑ Jaspal Patil; Alexander Schwab; Juerg Nussberger; Thomas Schaffner; Juan M. Saavedra; Hans Imboden (2010). "Intraneuronal angiotensinergic system in rat and human dorsal root ganglia". Regulatory Peptides. 162 (1-3): 90–98. doi:10.1016/j.regpep.2010.03.004.
- ↑ Jaspal Patil; Alexander Schwab; Juerg Nussberger; Thomas Schaffner; Juan M. Saavedra; Hans Imboden (2010). "Intraneuronal angiotensinergic system in rat and human dorsal root ganglia". Regulatory Peptides. 162 (1-3): 90–98. doi:10.1016/j.regpep.2010.03.004.
- ↑ Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P (2013). "Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons.". Eur J Pain. 17 (7): 1012–26. doi:10.1002/j.1532-2149.2012.00269.x.
- ↑ Steckelings UM, Kaschina E, Unger T (2005). "The AT2 receptor--a matter of love and hate". Peptides. 26 (8): 1401–9. doi:10.1016/j.peptides.2005.03.010. PMID 16042980.
- ↑ Sumners C, Horiuchi M, Widdop RE, McCarthy C, Unger T, Steckelings UM (2013). "Protective arms of the renin-angiotensin-system in neurological disease". Clin Exp Pharmacol Physiol. 40 (8): 580–588. doi:10.1111/1440-1681.12137.
- ↑ Smith, Maree T.; Woodruff, Trent M.; Wyse, Bruce D.; Muralidharan, Arjun; Walther, Thomas (2013). "A Small Molecule Angiotensin II Type 2 Receptor (AT2R) Antagonist Produces Analgesia in a Rat Model of Neuropathic Pain by Inhibition of p38 Mitogen-Activated Protein Kinase (MAPK) and p44/p42 MAPK Activation in the Dorsal Root Ganglia". Pain Medicine. 14 (10): 1526–4637. doi:10.1111/pme.12157.
- ↑ Basbaum AI, Bautista DM, Scherrer G, Julius D (2013). "Cellular and molecular mechanisms of pain". Cell. 139 (2): 267–284. doi:10.1016/j.cell.2009.09.028.
- ↑ WO patent 2011088504, MCCARTHY THOMAS DAVID; BAKER ANDREW RAINSFORD, "METHODS AND COMPOSITIONS FOR IMPROVED NERVE CONDUCTION VELOCITY", published 2006-06-29
- ↑ Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th (2002). "cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation". Neuron. 35 (4): 721–31. doi:10.1016/S0896-6273(02)00802-4.
- ↑ Bhave G, Zhu W, Wang H, Brasier DJ, Oxford GS, Gereau RW 4th (2002). "cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation". Neuron. 35 (4): 721–31. doi:10.1016/S0896-6273(02)00802-4.
- ↑ Spinifex Pharmaceuticals Pty Limited (2011). "CLINICAL STUDY PROTOCOL: Protocol No. EMA401-003". External link in
|title=(help) - ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ Anand U, Facer P, Yiangou Y, Sinisi M, Fox M, McCarthy T, Bountra C, Korchev YE, Anand P (2013). "Angiotensin II type 2 receptor (AT2 R) localization and antagonist-mediated inhibition of capsaicin responses and neurite outgrowth in human and rat sensory neurons.". Eur J Pain. 17 (7): 1012–26. doi:10.1002/j.1532-2149.2012.00269.x.
- ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ Spinifex Pharmaceuticals Pty Limited (2011). "CLINICAL STUDY PROTOCOL: Protocol No. EMA401-003". External link in
|title=(help) - ↑ Andrew S C Rice; Robert H Dworkin; Tom D McCarthy; Praveen Anand; Chas Bountra; Philip I McCloud; Julie Hill; Gary Cutter; Geoff Kitson; Nuket Desem; Milton Raff (2004). "EMA401, an orally administered highly selective angiotensin II type 2 receptor antagonist, as a novel treatment for postherpetic neuralgia: a randomised, double-blind, placebo-controlled phase 2 clinical trial". The Lancet. 383: 1637–1647. doi:10.1016/S0140-6736(13)62337-5.
- ↑ US patent 5246943A, BLANKLEY CLIFTON J; HODGES JOHN C, "Substituted 1,2,3,4-Tetrahydroisoquinolines with Angiotensin II Receptor Antagonist Properties", published 1993-07-21
- ↑ WO patent 2012010843A1, MCCARTHY THOMAS DAVID; KELLY PETER MICHAEL, "SALT AND SOLVATES OF A TETRAHYDROISOQUINOLINE DERIVATIVE", published 2013-01-26