Ectomycorrhiza
.jpg)
An ectomycorrhiza (Gk. ἐκτός, ektos, "outside;" μυκός, mykós, "fungus;" ριζα, riza, "roots;" pl. ectomycorrhizas or ectomycorrhizae, abbreviated EcM) is a form of symbiotic relationship that occurs between a fungal symbiont and the roots of various plant species. The mycobiont tends to be predominantly from the phyla Basidiomycota and Ascomycota, although a few are represented in the phylum Zygomycota.[1] Ectomycorrhizas form between fungi and the roots of around 2% of plant species.[1] These tend to be composed of woody plants, including species from the birch, dipterocarp, myrtle, beech, willow, pine and rose families.[2]
Unlike other mycorrhizal relationships, such as arbuscular mycorrhiza and ericoid mycorrhiza, ectomycorrhizal fungi do not penetrate their host’s cell walls. Instead, they form an entirely intercellular interface, consisting of highly branched hyphae forming a latticework between epidermal and cortical root cells, known as the Hartig net.
Ectomycorrhizas are further differentiated from other mycorrhizas by the formation of a dense hyphal sheath, known as the mantle, surrounding the root surface.[3] This sheathing mantle can be up to 40 µm thick, with hyphae extending up to several centimeters into the surrounding soil. This hyphal network aids in water and nutrient uptake often helping the host plant to survive adverse conditions,[2] and in exchange, the fungal symbiont is provided with access to carbohydrates.
Many EcM fungal fruiting bodies are well known. These include the economically important and edible truffle (Tuber) and the deadly death caps and destroying angels (Amanita). They also form on many common temperate forest trees, such as pines (Pinus), oaks (Quercus), willows (Salix), Douglas firs (Pseudotsuga), eucalypts (Eucalyptus), beeches (Fagus) and birches (Betula).
There have been tremendous advances in research concerning ectomycorrhizal identification and ecological importance over the past few years. This has led to a more complete understanding of the intricate and varied roles ectomycorrhizas play in the ecosystem. These advances in knowledge have led to increased applicability in areas such as ecosystem management and restoration, forestry and agriculture.
Evolution
Mycorrhizal symbioses, in general, are ubiquitous in terrestrial ecosystems, and it is possible that these associations helped to facilitate land colonization by plants. Paleobiological and molecular evidence suggest that arbuscular mycorrhizas (AM), in particular, originated at least 460 million years ago.[4]
EcM plants and fungi exhibit a wide taxonomic distribution and are similarly present across all continents (apart from Antarctica), suggesting the EcM symbiosis has ancient evolutionary roots, as well.[1] Pinaceae represents the oldest extant plant family in which symbiosis with EcM fungi occurs,[5] and fossils from this family date back to 156 million years ago.[6]
A popular theory proposed by Read[7] postulates that habitat type and the distinct functions of different mycorrhizas help determine the particular symbiosis that will become predominant. In this theory, EcM symbioses evolved in relatively productive ecosystems, such as boreal forests, but in which nutrient cycling could still be limiting. In this scenario, ectomycorrhizas are a somewhat intermediate form, having greater mineralization capacities than arbuscular mycorrhizas and less so than types such as ericoid mycorrhizas. This is supported by several studies, some of which also purport arbuscular mycorrhizas to be the ancestral trait. According to this data, many non-mycorrhizal and other mycorrhizal forms represent evolutionary specializations.[8][9]
Paleobiology
Fungi tend to be composed of soft tissues, making fossilization difficult and the discovery of fungal fossils incredibly rare. This is compounded by the microscopic size and ephemeral nature of ectomycorrhizas and their structure. However, some exquisitely preserved specimens have been discovered in the middle Eocene Princeton Chert of British Columbia. These ectomycorrhizal fossils show clear evidence of a Hartig net, mantle and hyphae, demonstrating well-established EcM associations at least 50 million years ago.[6]
It is widely known from the fossil record that the more common arbuscular mycorrhizas formed long before more derived associations, and thus represent an ancestral condition.[4][8][9] Ectomycorrhizas, forming with an array of conifers and angiosperms, may have evolved along with the diversification of plants. Thus, it is possible that arbuscular mycorrhizas were a driving force in the plant colonization of land as an expansive new niche, while ectomycorrhizas acted to spur further speciation due to the change of earth’s climate to more seasonal and arid, or perhaps simply in response to nutritionally deficient habitats.[9][10]
Molecular studies
According to molecular and phylogenetic analyses of fungal lineages, it appears that EcM fungi have evolved multiple times from humus and wood saprotrophic ancestors, with little reversion.[1] It is suggested that the EcM condition has evolved and persisted numerous times independently from non-EcM ancestors. These claims range from more conservative estimates of 7-16[5][11][12] to approximately 66 origins of EcM associations.[1]
Some studies suggest that reversals back to the ancestral free-living condition have occurred,[11] but this evidence has been extensively challenged because: 1) the data exhibits taxa sampling bias and model dependency, 2) most non-mycorrhizal taxa lie within strongly AM clades, rather than EcM ones, and 3) the derived EcM condition is specialized, and would likely have given an ecological advantage that reversion to saprotrophy would not have.[9][12] Furthermore, Hibbett and Matheny performed Bayesian relaxed molecular clock analyses yielding results that indicate that an ancestral EcM condition that was subsequently lost multiple times is simply not parsimonious. These reversals to a saprotrophic mode are impractical given that the host plants involved in the EcM symbioses (Pinaceae and rosids) had not yet evolved when the studied class Agaricomycetes first appeared.[5]
Morphology
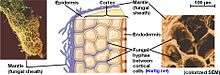
As suggested by the name, much of the biomass of the mycosymbiont lies exterior to the plant root. The fungal structure is composed primarily of three parts: 1) the intraradical hyphae making up the Hartig net, 2) the mantle that forms a sheath surrounding the root tip and 3) the extraradical hyphae and related structures that spread throughout the soil matrix.
Hartig net
The hartig net is formed by an ingrowth of hyphae (often originating from the inner part of the surrounding mantle) into the root of the plant host. The hyphae making up the Hartig net penetrate and grow in a transverse direction to the axis of the root,[13] and thus form a network between the outer cells of the root axis. This region of juxtaposition is where nutrient and carbon exchange occurs.[14]
The depth of penetration differs between species, with some being superficially confined to the epidermis (such as in Eucalyptus and Alnus), whereas in other cases the hyphae extend to the cortical cells or to the endodermis (as is the case in most gymnosperms).[2] In many epidermal types, radial elongation of epidermal cells occurs. However, this is largely absent in the cortical type, suggesting different strategies of increasing surface contact among species.[2]
Mantle
Enveloping the root, and often containing more biomass than the Hartig net interface, is a hyphal sheath known as the mantle. There exists considerable variation in the structure of the mantle, ranging from a loose network of hyphae to a structured and stratified arrangement of tissue. Often, these layers resemble plant parenchyma tissue and are referred to as pseudoparenchymatous.[14]
Due to the encapsulating nature of the mantle, the root of the plant symbiont is often affected developmentally. EcM fungal partners characteristically suppress root hair development of the host plant with which they are involved.[14] Also, through the induction of altered levels of cytokinins in the phytosymbiont, root branching can be increased.[15] These branching patterns can become so extensive that a single consolidated mantle can envelop many root tips at a time, yielding tuberculate or coralloid ectomycorrhizas.[14]
Often, the mantles of different EcM pairs display characteristic traits such as color, extent of branching, and degree of complexity. While fruiting bodies may provide a useful diagnostic in mycobiont identification, such structures and their connection to the zone of contact are not always available.[2] With the advent of more precise genetic techniques, these traits of the mantle are often used in tandem with molecular analyses to more easily determine the identity of the mycorrhizal association.[14]
Extraradical hyphae and linkage

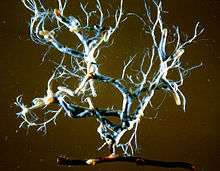
Extraradical hyphae extend outward from the mantle into the soil, fulfilling the role of the suppressed root hairs by increasing the surface area of the colonized root. These hyphae can spread out singly, or in an aggregate arrangement known collectively as a rhizomorph. Much as the Hartig net and mantle, composite hyphal organs can display a wide range of structural difference. Some rhizomorphs are simply parallel, linear collections of hyphae. Others yield more complex organization such as aggregates where the central hyphae possess enlarged diameters, or exhibiting apically extending hyphae that superficially resemble meristematic activity.[2]
The extramatrical mycelia of ectomycorrhizas function largely as transport structures. As such, they are often able to spread considerable distances to maintain a large contact area with the soil.[16] Some studies have even shown a relationship between nutrient transport rates and the degree of rhizomorph organization.[2][17] Often, the rhizomorphs of different EcM associations fall under different classifications of organization types and exploration strategies based on structure and growth within the soil.[16] These are capable of serving as useful diagnostic tools in identification, as well.
The hyphae extending outward into the soil from one ectomycorrhiza can serve as a source of EcM infection to other nearby plants. This can lead to the formation of common mycorrhizal networks (CMNs), which experiments have shown to culminate in the sharing of carbon and nutrients among the connected host plants in vitro.[18][19] In a field study at a site of primary succession on Mount Fuji, Nara demonstrated a likely alleviation of nitrogen competition among seedlings involved in CMNs, as well.[20]
Although physiological evidence of linkage is difficult to demonstrate, some experiments have been performed where carbon-14 was added to a particular tree and the labeled carbon was later detected in nearby plants and seedlings.[21] A more definitive study by Simard et al. demonstrated a bidirectional carbon transfer between Betula papyrifera and Pseudotsuga menziesii, primarily through the direct hyphal pathway.[22] This study also suggested that a source-sink relationship exists under field conditions to regulate this carbon transfer.[22] However, these networks are largely limited by the vegetative matrix and by whether or not neighboring plants are compatible with existing fungal networks. This becomes important when evaluating the ability of plants to exploit the benefits of established ectomycorrhizal linkages.[21]
It is hypothesized that these CMNs could be involved with other ecological processes stemming from a shared nutrient connection, such as seedling establishment, forest succession and other plant-plant interactions. There is little in the way of definitive data dealing with these proposed functions; however, some promising research exists for some arbuscular mycorrhizas.[23][24]
Fruiting bodies

A fourth section, which can be thought of as an extension of the extraradical hyphae, is the reproductive fruiting body of the EcM fungus. These structures vary widely in their morphology, although certain aspects are relatively conserved among species. The fungal cell walls are typically composed of complex carbohydrates, and a great deal of nitrogen is often bound in these cell walls and spores.[25]
Unlike most AM (arbuscular mycorrhizal) fungi, EcM fungi reproduce sexually and produce macroscopic sporocarps in a wide variety of forms.[1] It is often necessary for the fungal species involved in the symbiosis to form ectomycorrhizal relationships in order to complete their life cycles through the formation of fruiting bodies.
The structures of the fruit bodies of many species take on classic, well-recognized shapes. These are often hallmark forest constituents such as epigeous mushrooms and hypogeous truffles. Most of these produce microscopic propagules of about 10 μm that can disperse over large distances by way of various vectors, from wind to mycophagous animals.[26] Animals are often drawn to hypogeous fruiting bodies because they are rich in nutrients such as nitrogen, phosphorus, minerals and vitamins.[14] However, some sources state these nutritive properties are overstated, and it is more likely due to availability at specific times of the year and ease of harvest.[25]
Surveys of fruiting bodies are commonly used to assess community composition and richness in many studies. However, there are many problems with this method, including ephemeral nature of the sporocarps, difficulty in detecting certain forms (such as hypogeous structures), and infrequency of formation in many species.[27] Thus, along with advances in easier and more accurate molecular techniques, it has fallen out of favor when conducting studies where high accuracy and/or resolution is needed.
Physiology
Presymbiosis
In order to form an ectomycorrhizal connection, the fungal hyphae, originating from a soil propagule or an established mycorrhiza, must first grow towards the plant’s roots. Afterwards, it must envelope and penetrate the root cap cells and infect them, thus allowing the symbiotic Hartig net and associated structures to form. Both the plant and fungal partners must engage in a precise developmental sequence that allows the necessary genes in each symbiont to be expressed in order to carry out these actions.
In a study concerning a Tilia americana-Tuber borchii ectomycorrhiza, 29 volatile organic compounds were produced only during the interaction phase between the two partners, suggesting some of these compounds could play a role in the early stages of the formation of the ectomycorrhiza.[28] Another study on the same ectomycorrhizal association by Menotta et al. showed 58 genes were differentially expressed during the pre-contact phase, most of which were involved in secretory, apical growth, and infection processes.[29] Thus, there appears to be a complex set of molecular changes that occurs even before the fungus and host plant make contact.
From the plant hosts, critical metabolites are released into the rhizosphere that are capable of triggering basidiospore germination, growth of hyphae towards the root, and the early steps of the EcM formation.[30] These exudates can include, but are not limited to, flavonoids, diterpenes, cytokinins, hormones and other nutrients. Some host-released metabolites have been shown to stimulate fungal growth in Pisolithus, modify the branching angle of hyphae, and cause the accumulation of certain fungal molecules that tend to be present in higher quantities during mycorrhiza development.[30]
A few fungal genes that appear to be expressed before plant contact include PF6.2 and ras from Laccaria bicolor, and ras from Pisolithus. These discoveries offer further evidence for the induction of fungal genes by diffusible elicitors present in the soil.[30]
Symbiosis
Upon the connection of fungal hyphae and root cap cells, growth must continue inwards to the epidermal cells wherein the hyphae multiply to form layers that will eventually yield a mature mantle. In some associations, such as Eucalyptus globulus–Pisolithus tinctorius, up to 65 genes can be responsible for the production of the fungal mantle. Upregulation of genes responsible for translation and cell growth, such as eIF4A, and those responsible for membrane synthesis and function, such as the multigene SRAP32 family and hydrophobins, are quite common in EcM mycobionts.[31] Hilbert and Martin discovered 10 out of 520 polypeptides recorded during their study were unique to the symbiotic condition and subsequently these symbiosis-related (SR) proteins were termed “ectomycorrhizins”.[32]
Hilbert et al. showed that major changes in polypeptide synthesis occurred after only a few hours of colonization by the fungus.[33] Seven of the aforementioned ectomycorrhizins were detected, as well as a polypeptide cleansing event as several plant and fungal polypeptides underwent a sharp decrease. Comparative analyses of the mRNAs from both free-living mycelium and the EcM mycobionts showed many changes in gene expression, including SRAP32.[2]
At the root tip interface, researchers have observed a homologue for a phosphatidylserine decarboxylase (Psd) gene that is consistently upregulated compared to the extraradical mycelium. This could allow for new membranes to form at the symbiotic interface, which, in turn, could help explain the development of various permeases that occur in these locations. These new membranes could be largely responsible for nutrient transport from the fungal mycelium to the plant host.[34]
In some cases, the addition of fungal exudates alone partially mimicked the effect of the mantle in terms of root proliferation, root hair development and dichotomous branching.[30] Some of these exudates act synergistically upon hyphal morphology, as with rutin (a flavonol) and zeatin (a cytokinin). However, some other metabolites act antagonistically, demonstrated by root development stemming from the release of hypaphorine and indole-3-acetic acid (IAA). IAA must be tightly regulated in ectomycorrhizal symbioses due to its ability to inhibit root development above certain concentrations.[30]
The Hartig net initially forms from the fully differentiated inner layer of the mantle, and penetration occurs in a broad front oriented transversally to the root axis, rather than from a single hyphae.[13] As the hyphae contact the root cells and digest through the apoplastic space, some spruce (Picea abies) and eucalyptus (Eucalyptus globulus) cells have been shown to produce chitinases and peroxidases that could inhibit Hartig net formation.[30] Some plant cells exhibit transcripts for stress- and defense-related proteins such as pathogenesis-related and hypersensitive response-induced proteins.[2] However, extensive root colonization still occurs in these plants, and the association does not seem to be met with massive cell death associated with pathogen limitation or defense gene activation. In fact, as the relationship develops in a compatible manner, these hallmarks of resistance seem to diminish by about day 21 after colonization.[2] Thus, there must be some sort of defense response suppression by the EcM fungi, though details concerning its nature and mechanisms have yet to be determined.
This is well illustrated by the monosaccharide uptake system in Amanita muscaria. Carbon uptake requires a transporter, AmMST1, that is only expressed when the fungus is cultured at low glucose levels in multiple mycorrhizal associations. This expression, and increased import of monosaccharides by the fungus, is met with an increase in the supply of photoassimilates from the plant host. In the reverse direction, phosphoenolpyruvate carboxylase (PEPC) moderates the assimilation of ammonium and the transport of amino acids from fungus to plant.[31][34]
Nutrient uptake and exchange
Nitrogen is a crucial component of plant biochemistry, being involved in such integral compounds as chlorophyll, enzymes and amino acids. However, in a vast majority of terrestrial ecosystems it is a limiting nutrient, and readily available nitrogen is in short supply compared to the recalcitrant organic forms that are often shielded from rapid breakdown. Thus, the formation of ectomycorrhizal associations offers an extremely beneficial solution by allowing for the greater exploratory capacity of fungal hyphae, as well as the more efficient acquisition of nitrogen from reserves contained in the organic horizon.[14][35]
There is evidence that shows that glutamine is transferred across the root interface, following the assimilation of ammonium by the fungus. However, the pathways responsible for this conversion are largely unknown, though there are several plausible hypotheses.[36] It is also important to note that the net transfer of nutrients to plants requires three transport components: 1) the soil-fungus interface, 2) the fungus-apoplast interface, and 3) the apoplast-root cell interface.[35]
As the hyphae of the Hartig net region become more densely packed, they press against the cell walls of the phytobiont’s root cells. Often the fungal and plant cell walls become almost indistinguishable where they meet, thus forming a homogenous interfacial matrix through which nutrients can easily disperse.[37] The tips of the branched hyphae contain dense cytoplasm with a high concentration of mitochondria and rough endoplasmic reticulum. This arrangement is stretched in the direction of hyphal growth indicating that the transfer of nutrients between fungus and plant is localized in this area of contact.[38] ATPase activity in both fungal and plant plasma membranes at the Hartig net indicate a cooperatively bidirectional nutrient exchange.[37]
Due to a lack of septate hyphae, a coenocytic, transfer cell-like structure characterizes the Hartig net of many ectomycorrhizas, which facilitates interhyphal transport.[35] Wall ingrowths in Pisonia, for example, resemble those found in other plant species where transfer cells yield high rates of nutrient transport between apoplast and symplast.[35] Overall, ectomycorrhizal fungi receive approximately 15% of the net primary production and can provide up to 86% of a host’s nitrogen needs.[26] Carbon allocation has been shown to be correlated with growth rates and nutrient availability. Belowground allocation is highest when nutrient availability is low and when growth rates are reduced.[39] Phosphorus is another typically limiting nutrient in many terrestrial ecosystems. Evidence suggests that phosphorus is transferred largely as inorganic orthophosphate.[37] Some mat-forming ectomycorrhizas contain ribonucleases capable of rapidly hydrolyzing DNA in order to obtain phosphorus from nuclei.[35]
Some studies have shown that nitrogen eutrophication decreases the amount of carbon allocation to soil biota over longer periods of time.[40][41] This can pose problems to the mycobionts, whose production of sporocarps later in the growing season is totally dependent upon the allocation of photosynthates from host plants.[40] Unlike the major community shifts that can occur in sporocarp responses to nitrogen addition, root tips and soil hyphae respond in a considerably subtler manner in the short term. However, over longer periods of time, eutrophication can cause major shifts in the dominant fungi and dramatically alter belowground diversity.[42]
Non-nutritional benefits
Extraradical hyphae, and rhizomorphs in particular, also offer invaluable transport of water in many species. Often these develop into specialized runner hyphae and rhizomorphs capable of extending quite far from the host roots, thereby increasing the functional water access area.[43][44] The hyphal sheath enveloping the root tips also acts as a physical barrier shielding plant tissues from pathogens and predators. Furthermore, there has been evidence suggesting that secondary metabolites of fungal partners are capable of acting as biochemical defense mechanisms against pathogenic fungi, nematodes and bacteria that may try to infect the mycorrhizal root.[14] Many studies also show that EcM fungi are capable of providing tolerance to soils with high concentrations of heavy metals,[45][46][47] salts,[48][49] radionuclides and organic pollutants.[14]
Ectendomycorrhiza
Although EcM fungal hyphae form the Hartig net outside of root cells, penetration of plant cortical cells occasionally occurs. Many species of ectomycorrhizal fungi are capable of forming mycorrhizas with other plant species where this penetration is the normal mode of mycorrhizal formation. These associations represent a form of symbiosis intermediate to arbuscular mycorrhizas and ectomycorrhizas, termed ectendomycorrhizas. Like EcM fungi, both the mantle and Hartig net are present, though sometimes at reduced density. However, more like AM fungi, the hyphae penetrate the host’s root intracellularly. Thus, the same species of fungi can be categorized as ecto- or ectendomycorrhizas depending on the host species.[50]
Ecology
Biogeography and environmental gradients
Ectomycorrhizal fungi are found throughout boreal, temperate and tropical ecosystems, primarily among the dominant woody-plant-producing families (3.1.a).[26] In these more mesic environments supporting coniferous and mixed coniferous and deciduous forests, the EcM produce proteases and acid phosphatase enzymes to access organic forms of both nitrogen and phosphorus.[14]
Many of the fungal families most common in temperate forests (e.g. Russulaceae, Boletaceae, Thelephoraceae) are also quite widespread in the southern hemisphere and tropical dipterocarp forests. While there are differences between the fungal makeup of these different ecosystems, the ectomycorrhizal fungal component shows much greater similarities than the minimal overlap that occurs between dominant plant families in temperate and tropical forests (3.5.a).[51]
There is evidence to suggest that communities of EcM fungi differ across soil type gradients in a tropical system. However, the particular study notes that the mechanism driving this differentiation is not clear, and the variance could be in response to the soil physiochemical environment, plant community, or both (3.5.a). Other studies offer evidence to strengthen the idea that EcM communities are indeed affected by soil environment both in the field[52][53] and in the lab.[54][55]
Some studies indicate that ectomycorrhizal fungi might be at odds with the general latitudinal gradient of diversity (LGD). Data sets, free from inconclusive fruit-body surveys and largely relying on more accurate sequencing and microarray technologies, indicate that EcM fungi may be at enhanced diversity in the temperate zone.[26][56] This implies that many of the causal mechanisms proposed to explain the LGD pattern might be inapplicable, or in need of modification, in reference to EcM. Though this relationship is far from certain, there exist some hypotheses to explain the plausibility of this phenomenon: 1) EcM fungi may have evolved at higher latitudes with Pinaceae hosts, and are subsequently inferior at competition in tropical climates, 2) host lineages might be more diverse in temperate conditions, and well developed soil and soil horizons in temperate regions allow for higher niche differentiation and species accumulation, and 3) tropical EcM hosts are more sparsely distributed, yielding small isolated forest islands that may reduce the population sizes and subsequent richness of EcM fungi.[56]
This third hypothesis is expounded on in a study demonstrating that habitat size also plays an important role in determining the species richness and assemblage structure of ectomycorrhizal fungi, namely that richness is reduced in smaller and more isolated habitat areas. The same study determined that spatial turnover of soil fungi actually occurs on scales more similar to macro-organisms.[26]
Similar to studies concerning nitrogen eutrophication, EcM fungal makeup over an anthropogenic nitrogen gradient show similar trends. Species richness declined dramatically with increasing nitrogen inputs, with over 30 species represented at low nitrogen sites and only 9 at high nitrogen sites.[57] It is speculated that as nitrogen increases, taxa shift from those specialized for low nitrogen conditions to those specialized for phosphorus uptake in low phosphorus, high nitrogen, acidified conditions.[9][57]
Host specificity and community responses
Across most EcM host lineages, there appears to be low levels of specificity, as EcM plants tend to form symbioses with many distantly related fungi.[58] The benefits of this system are twofold: 1) seedlings are more likely to form mycorrhizas in a wide array of habitats, thus extending range and setting, and 2) EcM mycobionts can differ in their ability to access nutrients, thus allowing host plants better access to these minerals.[59] Many species of Alnus exhibit a very narrow range of fungal symbionts, but these fungi are not from related lineages. Thus it is the mycobionts that show phylogenetic specificity, not the alders.[59]
A major exception to the general rule outlined above is exemplified by mycoheterotrophic plants that utilize ectomycorrhizas for their carbon needs. These plants, from the subfamily Monotropoideae, exhibit high specificity for the EcM fungi they parasitize, although different monotrope species target a rather wide array of fungal lineages.[59]
While the plant hosts exhibit low specificity, EcM fungi exhibit various levels of specificity, and the costs and benefits to their specialization are not well understood.[60][61][62] A good example is the suilloid group, a monophyletic assemblage containing the genera Suillus, Rhizopogon, Gomphidius and others. This is the largest group that exhibits such an extreme degree of specificity, with almost all of its members forming ectomycorrhizas with members of the Pinaceae.[59] However, many other fungal groups exhibit a very broad host-range more akin to host lineages.[63][64]
Host plants that are taxonomically related show more similar EcM fungal communities than do taxa that are more distantly related.[65] Molecular phylogenetic studies have shown that fungi derived from a common ancestor are more likely to show host specificity to plants that are taxonomically related.[11][66] Host successional status may also play a role in determining EcM fungal communities, as well as affecting the number of EcM fungal species associated with an individual host species.[65] Other indirect factors can also play a role in the EcM fungal community, such as leaf fall and litter quality, which subsequently affect calcium levels and soil pH.[67] Even establishment timeframe of the host species can have an effect, with lower EcM fungal richness associated with hosts from a secondary forest than from a primary forest.[65]
The establishment of common mycelial networks is thought to have effects upon the plant community involved with them through their ectomycorrhizal connections. This can range from providing access to larger nutrient pools, mediating competition, and allowing resources and nutrients to be shared among individuals linked in this manner.[20][22][68]
Roles in invasion

Mycorrhizas have been regarded as the most prevalent symbiotic condition on earth, and as such they are essential to plant nutrition in terrestrial ecosystems. Thus, even alien plants often require mycorrhizal symbionts for the establishment and spread into foreign environments. Due to the low specificity of the vast majority of arbuscular mycorrhizas, AM plants often become invasive quickly and easily, and as such, the invasions are not necessarily accompanied by a simultaneous AM fungal invasion. However, because ectomycorrhizal symbioses present a range of specificities, exotic forestry has often relied upon the introduction of compatible EcM fungi to the foreign landscape in order to ensure the success of forest plantations and the like.[69]
This is most common in eucalypts and pines, which are obligate ectomycorrhizal trees in natural conditions.[69] This is evidenced by the struggle of establishment of pines in the southern hemisphere until the anthropogenic buildup of soil inoculums.[70] Similarly, Australian eucalypts and acacias have evolved in isolation from the EcM fungi associated with many other temperate trees such as Pinus and Quercus. Thus, much like pines in the southern hemisphere, many Eucalyptus plantations required inoculation by EcM fungi from their native landscape. In both cases, EcM networks allowed for the naturalization of the introduced species, followed quickly by competition for resources with native plants and invasion into novel habitats.[69]
Many EcM species co-invade without the help of human activity, however. Members of Pinaceae represent another prime example of this convention, often invading habitats along with specific EcM fungi from the genera Suillus and Rhizopogon.[60] There are, however, ectomycorrhiza-forming fungi with cosmopolitan distributions. These EcM fungi allow non-native plant species to form mutualisms that are not novel in environments that are, thus bypassing the need for co-invasion with specific EcM fungi from the native ecosystem.[60]
Dominant native plants are capable of inhibition of EcM fungi on the roots of neighboring plants through the release of chemical compounds or through competitive interactions.[71] Some invasive plants are capable of inhibiting the growth of native ectomycorrhizal fungi through similar mechanisms, especially if they become established and dominant. Invasive garlic mustard, Alliaria petiolata, and its allelochemical benzyl isothiocyanate were shown to inhibit the growth of three species of EcM fungi grown on white pine (Pinus strobus) seedlings.[72] Changes in EcM communities can have drastic effects on nutrient uptake and community composition of native trees, which can in turn have far-reaching ecological ramifications.[61]
Competition and other plant symbionts
Competition among EcM fungi is a well-documented case of soil microbial interactions.[73][74][75][76] In many experimental cases, the timing of colonization between competing EcM fungi determined which species was dominant. Namely, there was a priority effect that significantly favored the original colonists to be the most dominant, except in cases involving fungal species at a natural competitive disadvantage.[73][74] This disadvantage appears to be related to the proportion of root tips colonized, and those species incapable of colonizing a sufficient proportion of host roots do not typify this priority effect.[73]
Many other biotic and abiotic factors can mediate competition among EcM fungi, such as temperature, soil pH, soil moisture, host specificity, and competitor number.[74][75] The results of many studies concerning these factors indicate that these interactions are largely environmentally context-dependent. These aspects can often lead to “checkerboard” distribution patterns, where certain species occupy locations that are mutually exclusive of the other species.[74]
EcM communities continue to exhibit rare EcM fungal constituents that have not been excluded, despite intense competition. Thus, mechanisms must exist that maintain diverse levels of EcM fungi.[74] This coexistence can be summed up in four non-mutually exclusive possibilities illustrated by Bruns: niche partitioning, disturbance-related patch dynamics, density-dependent mortality and competitive networks.[77]
There is also some evidence for competition between EcM fungi and arbuscular mycorrhizal fungi. This is mostly noted in species, such as certain eucalypts, that are capable of hosting both EcM and AM fungi on their roots.[78] There is also some evidence in larger scale systems, such as pinyon woodlands, although it is hard to extricate effects of mycorrhizal interactions (if there are any) from those of simple resource competition.[79]
Some soil bacteria have been shown to have beneficial effects upon the establishment of ectomycorrhizal symbioses.[80][81][82] Some of these bacteria, known as Mycorrhiza Helper Bacteria (MHBs), have been shown to stimulate EcM formation, root and shoot biomass. The presence of higher levels of ergosterol in the soil indicate that MHBs may be promoting fungal growth, as well, thereby generating an increase in mycelial tissue and hyphae capable of exploring greater soil volumes.[80] The mechanisms by which these bacteria stimulate mycorrhizal formation are unclear. However, some mechanistic hypotheses include the softening of cell walls to make root cells more receptive, stimulation of short root formation in plants to allow for a higher probability of encounters with fungal propagules, and mediation of chemical elicitors involved in mutual recognition.[82] However, regardless of mechanism, it is becoming evident that bacteria are more ubiquitous than previously thought and could represent a third component of mycorrhizas (3.4.l).[83]
However, not all bacteria exhibit beneficial effects, and there exists some bacteria whose effects are quite opposite to those of MHBs, thus inhibiting ectomycorrhizal formation.[81]
Faunal interactions

Many ectomycorrhizal fungi are known to rely upon mammals for the dispersal of spores, particularly those fungi with hypogeous fruiting bodies. Many species of small mammals exhibit a high degree of mycophagy, ingesting a wide taxonomic range of fungi. These mammals are often drawn to hypogeous fruiting bodies because they are rich in nutrients such as nitrogen, phosphorus, minerals and vitamins. However, some sources state these nutritive properties are overstated, and it is more likely due to availability at specific times of the year, ease of harvest, and patchy nature of distribution.[25]
The spores of these fungi are dispersed either by the actions of being unearthed and broken apart, or by ingestion and subsequent excretion. Some studies even suggest that passage through an animal's gut promotes the germination of these spores, although it is by no means necessary for a majority of fungal species.[84][85] Regardless, the ability of these certain mammals to spread fungal spores is thus indirectly related to plant community structure, by way of the pivotal role that EcM fungi play in plant nutrition and productivity.[25]
Many other sporocarps are grazed upon by invertebrates such as mollusks and fly larvae, some of which are even tolerant to the toxic α-amanitin. Belowground, populations of nematodes and springtails are maintined by consumption of fungal tissue.[14] There are also interesting studies concerning EcM fungi and arthropods. The ectomycorrhizal fungus Laccaria bicolor has been found to lure and kill springtails to obtain nitrogen, some of which may then be transferred to the mycorrhizal host plant. In a study by Klironomos and Hart, eastern white pine inoculated with L. bicolor was able to derive up to 25% of its nitrogen from springtails.[86]
Of course, edible fungus plays a role in many societies throughout the world, as well. Many epigeous mushrooms are collected and consumed on a regular basis, and more recent commercial harvesting is beginning to play a larger economic role in certain locales.[87] Certainly, truffles (Tuber), porcinis (Boletus) and chanterelles (Cantharellus) are commonly known for, if not their taste and culinary importance, at least their billion dollar worldwide market.[88]
Plant production
Agriculture
Ectomycorrhizal fungi do not play a large role in agricultural and horticultural systems. Most of the economically relevant crop plants that form mycorrhizas tend to form them with arbuscular mycorrhizal fungi.[89] Many modern agricultural practices such as tillage, heavy fertilizers, and fungicides have extremely detrimental effects on crops’ associated mycorrhizas and on the surrounding ecosystem. Thus, it is possible that agriculture indirectly affects nearby ectomycorrhizal species and habitats, such as increased fertilization decreasing sporocarp production.[90][91]
Forestry
In commercial forestry, the transplanting of crop trees in new locales often requires an accompanying ectomycorrhizal partner. This is especially true of trees that have a high degree of specificity for their mycobiont, or trees that are being planted far from their native habitat among novel fungal species. This has been shown time and again in plantations involving obligate ectomycorrhizal trees, such as Eucalyptus and Pinus species.[69] Mass planting of these species often require human addition of inoculum from native EcM fungi in order for the trees to prosper.
Thus, these EcM fungi have to be species that are capable of being grown in bulk. After being added to various soil mixtures, the mutualism can begin as seedlings are grown in nurseries or plantations. This is already becoming quite commonplace, and there are many companies that are beginning to sell a variety of mycorrhizal inoculum, Pisolithus tinctorius being quite widespread among the EcM fungi.[90]
Sometimes ectomycorrhizal plantation species, such as pine and eucalyptus, are planted and promoted for their ability to act as a sink for atmospheric carbon. However, the ectomycorrhizal fungi of these species also tend to deplete soil carbon over relatively short periods of time.[92] Thus there is a great deal of mounting resistance to using tree plantations as general solutions to combatting rising carbon dioxide levels.[93]
Restoration
Ectomycorrhizas provide many benefits to their host plants, with enhanced nutrient uptake, growth and establishment in disturbed habitats ranked highly among them.[50] Thus, it seems logical that EcM fungi could be used in restoration projects aimed at re-establishing native plant species in ecosystems disrupted by a variety of issues.[94] In addition to providing a certain degree of protection to seedlings in harsh circumstances, such as increased salinity or heavy metal pollution, the fungi are also instrumental in improving soil quality.[94] They are able to achieve this through allowing the establishment of early vegetation and subsequent organic litter, preventing erosion, and binding soil particles together yielding stability and soil aggregation.[14] Since the disappearance of mycorhizal fungi from a habitat constitutes a major soil disturbance event, its re-addition is an important part of establishing vegetation and restoring habitats.[50]
Global change
Heavy metals
Heavy metals are well known toxic agents for living organisms. High soil concentrations of heavy metals such as zinc, copper, cadmium, lead, nickel, and chromium affect basic metabolic processes and can lead to cell damage and death. Ectomycorrhizal fungi are susceptible to heavy metal contamination. However, there seems to be widespread heavy metal tolerance in these fungi, with many species having the ability to colonize soils both with and without high heavy metal content. This said, there are a few examples of ecotypes associated with harsh soil chemistry,[95] indicating such inhospitable soils can lead to fungal evolutionary change.
Heavy metal excess interferes with basic cell functions, affecting the metabolism, growth, and differentiation of ectomycorrhizal fungi. High heavy metal concentrations may lead to the blocking of functional groups of important molecules such as enzymes, displacement and/or substitution of essential metals from molecules involved in cell processes, modification of molecule conformation and denaturation, as well as membrane disruption.[96] The effects of high heavy metal concentrations on ectomycorrhizal fungi vary by metal and fungal species and range from high fungal sensitivity to wide fungal tolerance. Overall, ectomycorrhizal fungi show high constitutive tolerance with many species being able to survive in toxic and non-toxic environments. However, there are cases of populations locally adapted to tolerate harsh chemical environments [95]
Fungi exhibit detoxification mechanisms that ensure heavy metal concentrations in their cells do not exceed a certain threshold. These mechanisms include reducing heavy metal uptake, promoting heavy metal sequestration and storage within the cell, and heavy metal excretion. Heavy metal uptake can be reduced by sorption and metabolic inactivation at the cell wall and apoplast level.[95] Ectomycorrhizal fungi also have the ability to bind considerable amounts of heavy metals,[95][97] yet it remains unclear if binding is an effective way to prevent heavy metals to enter fungal cells. Once inside the cell, heavy metals can be immobilized in organo-metal complexes, made soluble, transformed into metallothioneins, involved in metal sequestration and/or stored in vacuoles in chemically inactive forms. Antioxidant detoxification systems may also be in place, reducing the production of free radicals and protecting the fungal cell.[98][99] Fungi can export metals from the cytoplasm to the apoplast. This differential efflux efficiently discards heavy metals from the cell and is also known to occur in plants.[100] It has also been shown that ectomycorrhizal fungi growing in heavy metal rich soils often exhibit heavy metals in their sporocarps in addition to other mycelial tissue.[101] This binding of heavy metals to the cell wall, using components such as chitin and melanin, could possibly play a role in the mechanism determining ectomycorrhizal fungi heavy metal tolerance in general.[46]
Little is known about the environmental requirements or limitations of ectomycorrhizal fungi. However, many species are found to live in toxic and non-toxic environments and genetic differences between populations from such habitats have rarely been reported. This indicates widespread metal tolerance in these fungi. No metal-adapted endemic taxa have been documented so far.[97][102] There is however, evidence for community shifts associated with heavy metals,[103][104][105] with lower diversity associated with contaminated sites. Soils naturally rich in heavy metals, such as serpentine soils, on the other hand do not seem to imprint significant changes on ectomycorrhizal fungal communities.[106] In fact, the levels of fungal diversity in serpentine soils are comparable to those in non-serpentine soils and no serpentine endemics have so far been reported.
Although widespread tolerance seems to be the norm for ectomycorrhizal fungi, adaptive metal tolerance has been suggested for a few fungi such as Pisolithus tinctorius,[107] P. albus[108] and species in the genus Suillus.[109][110][111] Fungal ecotypes specialized to high levels of heavy metals have been found to be adapted to high levels of Al, Zn, Cd and Cu. Interestingly, a reduced number of species is known to be adapted to different metals with different ecotypes arising recurrently.[95] This suggests that some species are more prone to adapt to chemically inhospitable edaphic environments. Suillus luteus and S. bovinus are good examples, with known ecotypes adapted to Zn, Cd and Cu.[95][109][112][113] These differentiated populations accumulate lower heavy metal concentrations in their mycelia and in a slower fashion compared to sensitive ecotypes. More specifically, the accumulation of heavy metals in the fungal tissue is prevented through a mechanism that efficiently exports metals to the outside of the cell.
Pollution and Phytoremediation
One type of pollution that poses considerable challenges and threats to plants is the concentration of heavy metals in the soil. Although many metals are important nutrients in small quantities, such as copper, iron and zinc, in high concentrations they pose a particularly devastating risk due to their toxicities.[45] See Ectomycorrhizas and heavy metals for a description of how heavy metals affect ectomycorrhizal fungi.
Another problem faced by many plants is high soil salinity. One study shows that some EcM fungi are capable of improving salt tolerance in a species of poplar by altering leaf physiology. Though the symbiotic contact takes place at the root interface, the fungus was able to alter such leaf traits as concentration of nutrients and phytohormones, and ratios of fatty acids in order to combat leaf chlorosis and shedding.[114] In seagrape seedlings, the EcM fungus Scleroderma bermudense was able to alleviate salt stress. In the seagrape tissue, there was a decrease in both sodium and chlorine, but an increase in potassium and phosphorus, implying this trend might represent a mechanism to explain the observed tolerance.[48] Another study even identified 22 proteins differentially produced under salt stress of the EcM fungus Boletus edulis. They mostly concerned cellular processes such as metabolism, cell cycle control and stress tolerance, with 14 proteins being upregulated and 8 down.[49]
Many species of ectomycorrhizal fungi, most notably those from Cortinariaceae, are capable of becoming hyperaccumulators of radionuclides. This is similar to their ability to absorb heavy metals, though mechanisms are largely unresearched. In a study in Sweden, sporocarps of an ectomycorrhizal fungus contained ten times the concentration of radiocesium than the surrounding litter, while saprotrophic species exhibited nearly half that value.[115]
Some species are capable of decomposing persistent organic pollutants (POPs) as well, such as organochlorides and polychlorinated biphenyls (PCBs). Species such as Suillus variegatus and Paxillus involutus were able to mineralize 2,4-dichlorophenol both in axenic culture and in an EcM association with Pinus sylvestris.[116] The intact rhizosphere of Pinus taeda also exhibited the capacity to mineralize tetrachloroethylene under natural conditions.[116] The EcM fungi Radiigera atrogleba and Hysterangium garneri were capable of decomposing up to 80% of a particular PCB when tested.[14]
However, not every problem is alleviated by the presence of EcM fungi. There is some evidence that points to an inhibition in degrading recalcitrant pollutants such as polycyclic aromatic hydrocarbons. It is thought that these fungi take away nutrients from other degraders that would be better at degrading these compounds, therefore inhibiting their actions.[117]
Climate change
Climate change can induce a number of changes on the environment, and subsequently, ectomycorrhizal communities. Many of these studies are in their infancy, but it is clear that they often exhibit some effect. In some studies, elevated CO2 levels increased fungal mycelium growth due to increased carbon allocation[118] and increased EcM root colonization by 14%.[119] However, CO2 levels can affect different EcM associations quite differently, and many studies with negligible effects have also been performed.[120]
Increased temperatures also appear to affect EcM communities, though the results cover a range of responses. Some studies have shown that respiration is reduced in certain species in response to warming,[121] whereas others demonstrated increased total colonization of host plants.[52] Similarly, it appears that only some EcM species are affected by drought, as there are studies yielding results across the spectrum. However, many species provide protection against root desiccation and improve water uptake ability of the roots. In this sense, they provide a general benefit to plants during times of drought (though they may themselves may be affected over time).[120]
Regardless of how variably these EcM symbioses may change in response to the changing environmental conditions, it is clear that they do at least change. Thus, as more research is compiled, and as patterns and general effects emerge, we will have a better understanding of the no doubt critical consequences climate change has on EcM communities.
Conservation
As it becomes more apparent that belowground organisms and functions heavily influence forest productivity, recovery and stability, ectomycorrhiza are becoming a prime focus for conservation ecologists.[90] The recent decline of many species of EcM fungi in Europe has also allowed the importance of EcM disappearance to gain traction in more widespread conservation circles. Many factors are contributing to the decline, including reduced tree vitality, conversion of forests to other uses, pollution and acidification of forest soils.[90][122]
Conservation efforts need to be based on protecting species over their entire host range and habitat, not just at particular sites or with particular species.[90] Studies have shown that even in many different soil types and locations EcM fungal richness is relatively conserved, but that the community makeup of sites can still be quite different. Trees are often dominated by a few fungal strains, but these fungi are not the same on all trees in nearby areas, and show considerable spatial variation. This leads to the conclusion that conservation efforts should be aimed even at atypical sites, such as abiotically stressful locales, in order to conserve the full gamut of EcM fungal partners and plant host species.[27]
The growing importance of EcM fungi in the field of conservation is evidenced by the creation of the Northwest Forest Plan, which offers guidelines to maintain habitats and endangered species. This includes creating databases for endangered fungi and developing strategies to manage and protect them. Another example in a similar vein is the creation of the European Council for the Conservation of Fungi, which works with education about, and the documentation of, endangered fungi on the continent.[90] Organizations and groups like these are valuable in disseminating knowledge concerning conservation practices for scientists and the public. They also help compile solutions for fields like forest management that act to preemptively halt EcM declines.
In many cases, forest managers and scientists must take steps to ensure the health of economically important native forests, such as the maintenance of: 1) refuge plants and reservoir hosts after harvesting to preserve the EcM fungal community, 2) mature trees to provide seedlings with a diverse array of EcM fungi, and 3) old-growth stands with their more diverse macro- and microhabitats that support an abundance of EcM fungi.[123] Other strategies include the preservation of natural forest floor constituents and retention of woody debris and substrates. In one study concerning Douglas-fir seedlings, removal of forest floor debris and soil compaction decreased EcM fungal diversity and abundance by 60%.[124] Another study focused on the removal of pinegrass, and found that its removal had similar reductions in diversity and richness of EcM fungi.[22]
Some strategies, such as prescribed burns, have complicated ramifications due to conflicting evidence. Some claim that fires have negative effects on EcM survival and diversity,[125] while other show neutral, or even positive effects.[123][126] Clearly, different organisms and different community structures will react to such burns differently. With that in mind, these diverse effects are to be expected, and research on how specific communities could respond, rather than sweeping generalizations, would be much more beneficial in the long run.
Ex situ strategies for conservation of fungi are also currently under way, including ectomycorrhizal fungi, but a great deal of work is still needed. There are large culture collections maintained throughout the world; however, there are only approximately 11,500 species included. This represents only about 17% of known fungal species, and around 1% of the world’s estimated species of fungi.[127] A great deal of worldwide cooperation is needed in order to secure the fungal genetic resource for the future.
References
- 1 2 3 4 5 6 Tedersoo, Leho; May, Tom W.; Smith, Matthew E. (2010). "Ectomycorrhizal lifestyle in fungi: global diversity, distribution, and evolution of phylogenetic lineages" (PDF). Mycorrhiza. 20 (4): 217–263. doi:10.1007/s00572-009-0274-x. ISSN 0940-6360. PMID 20191371.
- 1 2 3 4 5 6 7 8 9 10 Smith, Sally E.; Read, David J. (26 July 2010). Mycorrhizal Symbiosis. Academic Press. ISBN 978-0-08-055934-6.
- ↑ Hock, Bertold (2012). Fungal Associations. Springer. ISBN 978-3-642-30826-0.
- 1 2 Simon, Luc; Bousquet, Jean; Lévesque, Roger C.; Lalonde, Maurice (1993). "Origin and diversification of endomycorrhizal fungi and coincidence with vascular land plants". Nature. 363 (6424): 67–69. doi:10.1038/363067a0. ISSN 0028-0836.
- 1 2 3 Hibbett, David S.; Matheny, P. Brandon (2009). "The relative ages of ectomycorrhizal mushrooms and their plant hosts estimated using Bayesian relaxed molecular clock analyses". BMC Biology. 7 (13). doi:10.1186/1741-7007-7-13. ISSN 1741-7007.
- 1 2 LePage, Ben A.; Currah, Randolph S.; Stockey, Ruth A.; Rothwell, Gar W. (1997). "Fossil ectomycorrhizae from the Middle Eocene" (PDF). American Journal of Botany. 84 (3): 410–412. doi:10.2307/2446014. ISSN 0002-9122.
- ↑ Read, David J. (1991). "Mycorrhizas in ecosystems". Experientia. 47 (4): 376–391. doi:10.1007/BF01972080. ISSN 0014-4754.
- 1 2 Fitter, A. H.; Moyersoen, B. (1996). "Evolutionary trends in root-microbe symbioses". Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 351 (1345): 1367–1375. doi:10.1098/rstb.1996.0120.
- 1 2 3 4 5 Wang, B.; Qiu, Y.-L. (2006). "Phylogenetic distribution and evolution of mycorrhizas in land plants" (PDF). Mycorrhiza. 16 (5): 299–363. doi:10.1007/s00572-005-0033-6. ISSN 0940-6360. PMID 16845554.
- ↑ Allen, Michael F. The ecology of mycorrhizae. Cambridge University Press, 1991.
- 1 2 3 Hibbett, David S.; Gilbert, Luz-Beatriz; Donoghue, Michael J. (2000). "Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes" (PDF). Nature. 407 (6803): 506–508. doi:10.1038/35035065.
- 1 2 Bruns, Thomas D.; Shefferson, Richard P. (2004). "Evolutionary studies of ectomycorrhizal fungi: recent advances and future directions" (PDF). Canadian Journal of Botany. 82 (8): 1122–1132. doi:10.1139/b04-021.
- 1 2 Blasius, D.; et al. (1986). "Hartig net structure and formation in fully ensheathed ectomycorrhizas". Nordic journal of botany. 6 (6): 837–842. doi:10.1111/j.1756-1051.1986.tb00487.x.
- 1 2 3 4 5 6 7 8 9 10 11 12 13 Dighton, J. "Mycorrhizae." Encyclopedia of Microbiology (2009): 153-162.
- ↑ Giron, David; et al. (2013). "Cytokinins as key regulators in plant–microbe–insect interactions: connecting plant growth and defence" (PDF). Functional Ecology. 27: 599–609. doi:10.1111/1365-2435.12042.
- 1 2 Agerer, Reinhard (2001). "Exploration types of ectomycorrhizae" (PDF). Mycorrhiza. 11 (2): 107–114. doi:10.1007/s005720100108.
- ↑ Kammerbauer, H; Agerer, R; Sandermann, H Jr (1989). "Studies on ectomycorrhiza. XXII. Mycorrhizal rhizomorphs of Thelephora terrestris and Pisolithus tinctorius in association with Norway spruce (Picea abies): formation in vivo and translocation of phosphate". Trees. 3: 78–84. doi:10.1007/bf00191537.
- ↑ Arnebrant, Kristina; et al. (1993). "Nitrogen translocation between Alnus glutinosa (L.) Gaertn. seedlings inoculated with Frankia sp. and Pinus contorta Doug, ex Loud seedlings connected by a common ectomycorrhizal mycelium". New Phytologist. 124 (2): 231–242. doi:10.1111/j.1469-8137.1993.tb03812.x.
- ↑ He, Xinhua; et al. (2006). "Rapid nitrogen transfer from ectomycorrhizal pines to adjacent ectomycorrhizal and arbuscular mycorrhizal plants in a California oak woodland". New Phytologist. 170 (1): 143–151. doi:10.1111/j.1469-8137.2006.01648.x.
- 1 2 Nara, Kazuhide (2006). "Ectomycorrhizal networks and seedling establishment during early primary succession". New Phytologist. 169 (1): 169–178. doi:10.1111/j.1469-8137.2007.02016.x.
- 1 2 Amaranthus, M. P.; Perry, D. A. (1994). "The functioning of ectomycorrhizal fungi in the field: linkages in space and time". Plant and soil. 159 (1): 133–140. doi:10.1007/BF00000102.
- 1 2 3 4 Simard, Suzanne W.; et al. (1997). "Net transfer of carbon between ectomycorrhizal tree species in the field" (PDF). Nature. 388 (6642): 579–582. doi:10.1038/41557.
- ↑ Babikova, Zdenka; et al. (2013). "Underground signals carried through common mycelial networks warn neighbouring plants of aphid attack" (PDF). Ecology Letters. 16: 835–843. doi:10.1111/ele.12115.
- ↑ Xie, L. J.; et al. (2012). "Disease resistance signal transfer between roots of different tomato plants through common arbuscular mycorrhiza networks". The journal of applied ecology. 23 (5): 1145.
- 1 2 3 4 Johnson, Christopher N (1996). "Interactions between mammals and ectomycorrhizal fungi". Trends in Ecology & Evolution. 11 (12): 503–507. doi:10.1016/S0169-5347(96)10053-7.
- 1 2 3 4 5 Peay, Kabir G.; et al. (2007). "A strong species–area relationship for eukaryotic soil microbes: island size matters for ectomycorrhizal fungi" (PDF). Ecology Letters. 10 (6): 470–480. doi:10.1111/j.1461-0248.2007.01035.x.
- 1 2 Gehring, Catherine A.; et al. (1998). "Ectomycorrhizal fungal community structure of pinyon pines growing in two environmental extremes" (PDF). Ecology. 79 (5): 1562–1572. doi:10.1890/0012-9658(1998)079[1562:efcsop]2.0.co;2.
- ↑ Menotta, Michele; et al. (2004). "Headspace solid‐phase microextraction with gas chromatography and mass spectrometry in the investigation of volatile organic compounds in an ectomycorrhizae synthesis system" (PDF). Rapid communications in mass spectrometry. 18 (2): 206–210. doi:10.1002/rcm.1314.
- ↑ Menotta, M.; et al. (2004). "Differential gene expression during pre-symbiotic interaction between Tuber borchii Vittad. and Tilia americana L.". Current genetics. 46 (3): 158–165. doi:10.1007/s00294-004-0518-4.
- 1 2 3 4 5 6 Martin, Francis, et al. "Developmental cross talking in the ectomycorrhizal symbiosis: signals and communication genes." New Phytologist 2001; 151(1): 145-154.
- 1 2 Egerton-Warburton, L. M.; et al. (2003). "Mycorrhizal fungi". Encyclopedia of Soils in the Environment.
- ↑ Hilbert, J. L.; Martin, F. (1988). "Regulation of gene expression in ectomycorrhizas". New phytologist. 110 (3): 339–346. doi:10.1111/j.1469-8137.1988.tb00270.x.
- ↑ Hilbert, Jean-Louis; Costa, Guy; Martin, Francis (1991). "Ectomycorrhizin synthesis and polypeptide changes during the early stage of eucalypt mycorrhiza development" (PDF). Plant Physiology. 97 (3): 977–984. doi:10.1104/pp.97.3.977.
- 1 2 Morel, Mélanie; et al. (2005). "Identification of genes differentially expressed in extraradical mycelium and ectomycorrhizal roots during Paxillus involutus-Betula pendula ectomycorrhizal symbiosis". Applied and Environmental Microbiology. 71 (1): 382–391. doi:10.1128/aem.71.1.382-391.2005.
- 1 2 3 4 5 Chalot, Michel; Brun, Annick (1998). "Physiology of organic nitrogen acquisition by ectomycorrhizal fungi and ectomycorrhizas". FEMS Microbiology Reviews. 22 (1): 21–44. doi:10.1111/j.1574-6976.1998.tb00359.x.
- ↑ Smith, S. E.; Smith, F. A. (1990). "Tansley review No. 20. structure and function of the interfaces in biotrophic symbioses as they relate to nutrient transport". New Phytologist. 114: 1–38. doi:10.1111/j.1469-8137.1990.tb00370.x.
- 1 2 3 Smith, S. E.; et al. (1994). "Nutrient transport in mycorrhizas: structure, physiology and consequences for efficiency of the symbiosis" (PDF). Plant and Soil. 159 (1): 103–113. doi:10.1007/BF00000099. JSTOR 42939411.
- ↑ Kottke, I.; Oberwinkler, F. (1987). "The cellular structure of the Hartig net: coenocytic and transfer cell‐like organization". Nordic journal of botany. 7 (1): 85–95. doi:10.1111/j.1756-1051.1987.tb00919.x.
- ↑ Hobbie, Erik A (2006). "Carbon allocation to ectomycorrhizal fungi correlates with belowground allocation in culture studies" (PDF). Ecology. 87 (3): 563–569. doi:10.1890/05-0755.
- 1 2 Högberg, Mona N.; et al. (2010). "Quantification of effects of season and nitrogen supply on tree below‐ground carbon transfer to ectomycorrhizal fungi and other soil organisms in a boreal pine forest". New Phytologist. 187 (2): 485–493. doi:10.1111/j.1469-8137.2010.03274.x.
- ↑ Wallander, H.; Ekblad, Alf; Bergh, J. (2011). "Growth and carbon sequestration by ectomycorrhizal fungi in intensively fertilized Norway spruce forests". Forest Ecology and Management. 262 (6): 999–1007. doi:10.1016/j.foreco.2011.05.035.
- ↑ Lilleskov, E. A.; Hobbie, E. A.; Horton, T. R. (2011). "Conservation of ectomycorrhizal fungi: exploring the linkages between functional and taxonomic responses to anthropogenic N deposition" (PDF). Fungal Ecology. 4 (2): 174–183. doi:10.1016/j.funeco.2010.09.008.
- ↑ Duddridge, JA; Malibari, A; Read, DJ (1980). "Structure and function of mycorrhizal rhizomorphs with special reference to their role in water transport". Nature. 287: 834–836. doi:10.1038/287834a0.
- ↑ Brownlee, C.; Duddridge, J. A.; Malibari, A.; Read, D. J. (1983). "The structure and function of mycelial systems of ectomycorrhizal roots with special reference to their role in forming inter-plant connections and providing pathways for assimilate and water transport". Plant and Soil. 71 (1-3): 433–443. doi:10.1007/BF02182684. ISSN 0032-079X.
- 1 2 Colpaert, Jan V.; et al. (2011). "How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution" (PDF). Annals of Forest Science. 68 (1): 17–24. doi:10.1007/s13595-010-0003-9.
- 1 2 Blaudez, Damien, Bernard Botton, and Michel Chalot. "Cadmium uptake and subcellular compartmentation in the ectomycorrhizal fungus Paxillus involutus." Microbiology 2000; 146(5): 1109-1117.
- ↑ Sell, Joachim; et al. (2005). "Contribution of ectomycorrhizal fungi to cadmium uptake of poplars and willows from a heavily polluted soil". Plant and soil. 277 (1-2): 245–253. doi:10.1007/s11104-005-7084-5.
- 1 2 Bandou, E.; et al. (2006). "The ectomycorrhizal fungus Scleroderma bermudense alleviates salt stress in seagrape (Coccoloba uvifera L.) seedlings". Mycorrhiza. 16 (8): 559–565. doi:10.1007/s00572-006-0073-6.
- 1 2 Liang, Y. U.; et al. (2007). "Proteome analysis of an ectomycorrhizal fungus Boletus edulis under salt shock" (PDF). Mycological Research. 111 (8): 939–946. doi:10.1016/j.mycres.2007.06.005.
- 1 2 3 Quoreshi, Ali M. "The use of mycorrhizal biotechnology in restoration of disturbed ecosystem." Mycorrhizae: Sustainable Agriculture and Forestry. Springer Netherlands, 2008. 303-320. doi:10.1007/978-1-4020-8770-7_13
- ↑ Peay, Kabir G.; et al. (2010). "Potential link between plant and fungal distributions in a dipterocarp rainforest: community and phylogenetic structure of tropical ectomycorrhizal fungi across a plant and soil ecotone". New Phytologist. 185 (2): 529–542. doi:10.1111/j.1469-8137.2009.03075.x. PMID 19878464.
- 1 2 Swaty, Randy L.; et al. (1998). "Temporal variation in temperature and rainfall differentially affects ectomycorrhizal colonization at two contrasting sites" (PDF). New Phytologist. 139 (4): 733–739. doi:10.1046/j.1469-8137.1998.00234.x.
- ↑ Toljander, Jonas F.; et al. (2006). "Species composition of an ectomycorrhizal fungal community along a local nutrient gradient in a boreal forest". New Phytologist. 170 (4): 873–884. doi:10.1111/j.1469-8137.2006.01718.x.
- ↑ Brearley, Francis Q (2006). "Differences in the growth and ectomycorrhizal community of Dryobalanops lanceolata (Dipterocarpaceae) seedlings grown in ultramafic and non-ultramafic soils". Soil Biology and Biochemistry. 38 (12): 3407–3410. doi:10.1016/j.soilbio.2006.05.012.
- ↑ Brearley, Francis Q.; et al. (2007). "How does light and phosphorus fertilisation affect the growth and ectomycorrhizal community of two contrasting dipterocarp species?.". Plant Ecology. 192 (2): 237–249. doi:10.1007/s11258-007-9325-6.
- 1 2 Tedersoo, Leho; Nara, Kazuhide (2010). "General latitudinal gradient of biodiversity is reversed in ectomycorrhizal fungi". New Phytologist. 185 (2): 351–354. doi:10.1111/j.1469-8137.2009.03134.x.
- 1 2 Lilleskov, Erik A.; et al. (2002). "Belowground ectomycorrhizal fungal community change over a nitrogen deposition gradient in Alaska" (PDF). Ecology. 83 (1): 104–115. doi:10.2307/2680124.
- ↑ Molina, Randy, Hugues Massicotte, and James M. Trappe. "Specificity phenomena in mycorrhizal symbioses: community-ecological consequences and practical implications." Mycorrhizal functioning: an integrative plant-fungal process (1992): 357-423.
- 1 2 3 4 Bruns, Thomas D.; Bidartondo, Martin I.; Taylor, D. Lee (2002). "Host specificity in ectomycorrhizal communities: what do the exceptions tell us?.". Integrative and Comparative Biology. 42 (2): 352–359. doi:10.1093/icb/42.2.352.
- 1 2 3 Dickie, Ian A.; et al. (2010). "Co‐invasion by Pinus and its mycorrhizal fungi". New Phytologist. 187 (2): 475–484. doi:10.1111/j.1469-8137.2010.03277.x.
- 1 2 Wolfe, Benjamin E.; Klironomos, John N. (2005). "Breaking new ground: soil communities and exotic plant invasion" (PDF). BioScience. 55 (6): 477–487. doi:10.1641/0006-3568(2005)055[0477:bngsca]2.0.co;2.
- ↑ Borowicz, Victoria A.; Juliano, Steven A. (1991). "Specificity in host-fungus associations: Do mutualists differ from antagonists?.". Evolutionary Ecology. 5 (4): 385–392. doi:10.1007/BF02214155.
- ↑ Diédhiou, Abdala Gamby; et al. (2010). "Multi‐host ectomycorrhizal fungi are predominant in a Guinean tropical rainforest and shared between canopy trees and seedlings". Environmental Microbiology. 12 (8): 2219–2232. doi:10.1111/j.1462-2920.2010.02183.x.
- ↑ Massicotte, H. B.; et al. (1999). "Diversity and host specificity of ectomycorrhizal fungiforest sites by five host species". Canadian Journal of Botany. 77 (8): 1053–1076. doi:10.1139/cjb-77-8-1053.
- 1 2 3 Ishida, Takahide A.; Nara, Kazuhide; Hogetsu, Taizo (2007). "Host effects on ectomycorrhizal fungal communities: insight from eight host species in mixed conifer–broadleaf forests". New Phytologist. 174 (2): 430–440. doi:10.1111/j.1469-8137.2007.02016.x.
- ↑ Den Bakker, Henk C.; et al. (2004). "Evolution and host specificity in the ectomycorrhizal genus Leccinum". New Phytologist. 163 (1): 201–215. doi:10.1111/j.1469-8137.2004.01090.x.
- ↑ Aponte, Cristina; et al. (2010). "Indirect host effect on ectomycorrhizal fungi: Leaf fall and litter quality explain changes in fungal communities on the roots of co-occurring Mediterranean oaks" (PDF). Soil Biology and Biochemistry. 42 (5): 788–796. doi:10.1016/j.soilbio.2010.01.014.
- ↑ Kennedy, P. G.; Izzo, A. D.; Bruns, T. D. (2003). "There is high potential for the formation of common mycorrhizal networks between understorey and canopy trees in a mixed evergreen forest". Journal of Ecology. 91 (6): 1071–1080. doi:10.1046/j.1365-2745.2003.00829.x.
- 1 2 3 4 Díez, Jesús. "Invasion biology of Australian ectomycorrhizal fungi introduced with eucalypt plantations into the Iberian Peninsula" (PDF). Issues in Bioinvasion Science. 2005: 3–15. doi:10.1007/1-4020-3870-4_2.
- ↑ Richardson, David M., ed. Ecology and biogeography of Pinus. Cambridge University Press, 2000.
- ↑ Walker, John F.; et al. (1999). "Suppression of ectomycorrhizae on canopy tree seedlings in Rhododendron maximum L. (Ericaceae) thickets in the southern Appalachians" (PDF). Mycorrhiza. 9 (1): 49–56. doi:10.1007/s005720050262.
- ↑ Wolfe, Benjamin E.; et al. (2008). "The invasive plant Alliaria petiolata (garlic mustard) inhibits ectomycorrhizal fungi in its introduced range". Journal of Ecology. 96 (4): 777–783. doi:10.1111/j.1365-2745.2008.01389.x.
- 1 2 3 Kennedy, Peter G.; Peay, Kabir G.; Bruns, Thomas D. (2009). "Root tip competition among ectomycorrhizal fungi: Are priority effects a rule or an exception?." (PDF). Ecology. 90 (8): 2098–2107. doi:10.1890/08-1291.1.
- 1 2 3 4 5 Kennedy, Peter. "Ectomycorrhizal fungi and interspecific competition: species interactions, community structure, coexistence mechanisms, and future research directions." New Phytologist 2010; 187(4): 895-910.
- 1 2 Mamoun, M.; Olivier, J. M. (1993). "Competition between Tuber melanosporum and other ectomycorrhizal fungi under two irrigation regimes". Plant and soil. 149 (2): 211–218. doi:10.1007/BF00016611.
- ↑ Villeneuve, Normand; Le Tacon, François; Bouchard, Daniel (1991). "Survival of inoculated Laccaria bicolor in competition with native ectomycorrhizal fungi and effects on the growth of outplanted Douglasfir seedlings". Plant and Soil. 135 (1): 95–107. doi:10.1007/BF00014782.
- ↑ Bruns, Thomas D. "Thoughts on the processes that maintain local species diversity of ectomycorrhizal fungi." The significance and regulation of soil biodiversity. Springer Netherlands, 1995. 63-73. PDF
- ↑ Chen, Y. L.; Brundrett, M. C.; Dell, B. (2000). "Effects of ectomycorrhizas and vesicular–arbuscular mycorrhizas, alone or in competition, on root colonization and growth of Eucalyptus globulus and E. urophylla". New Phytologist. 146 (3): 545–555. doi:10.1046/j.1469-8137.2000.00663.x.
- ↑ McHugh, Theresa A.; Gehring, Catherine A. (2006). "Below‐ground interactions with arbuscular mycorrhizal shrubs decrease the performance of pinyon pine and the abundance of its ectomycorrhizas". New Phytologist. 171 (1): 171–178. doi:10.1111/j.1469-8137.2006.01735.x.
- 1 2 Founoune, Hassna; et al. (2002). "Mycorrhiza helper bacteria stimulate ectomycorrhizal symbiosis of Acacia holosericea with Pisolithus alba". New Phytologist. 153 (1): 81–89. doi:10.1046/j.0028-646X.2001.00284.x.
- 1 2 Bowen, G. D.; Theodorou, C. (1979). "Interactions between bacteria and ectomycorrhizal fungi". Soil Biology and Biochemistry. 11 (2): 119–126. doi:10.1016/0038-0717(79)90087-7.
- 1 2 Garbaye, J (1994). "Tansley Review No. 76 Helper bacteria: a new dimension to the mycorrhizal symbiosis". New phytologist. 128 (2): 197–210. doi:10.1111/j.1469-8137.1994.tb04003.x.
- ↑ Bonfante, Paola; Anca, Iulia-Andra (2009). "Plants, mycorrhizal fungi, and bacteria: a network of interactions" (PDF). Annual Review of Microbiology. 63: 363–383. doi:10.1146/annurev.micro.091208.073504.
- ↑ Claridge, A. W.; et al. (1999). "Mycophagy by small mammals in the coniferous forests of North America: nutritional value of sporocarps of Rhizopogon vinicolor, a common hypogeous fungus". Journal of Comparative Physiology B. 169 (3): 172–178. doi:10.1007/s003600050208.
- ↑ Cork, Steven J.; Kenagy, G. J. (1989). "Nutritional value of hypogeous fungus for a forest-dwelling ground squirrel". Ecology. 70: 577–586. doi:10.2307/1940209.
- ↑ Klironomos, John N., and Miranda M. Hart. "Food-web dynamics: Animal nitrogen swap for plant carbon." Nature 2001; 410(6829): 651-652.
- ↑ Arnolds, Eef. "Conservation and management of natural populations of edible fungi." Canadian Journal of Botany 73.S1 (1995): 987-998.
- ↑ Yun, Wang; Hall, Ian R. (2004). "Edible ectomycorrhizal mushrooms: challenges and achievements" (PDF). Canadian Journal of Botany. 82 (8): 1063–1073. doi:10.1139/b04-051.
- ↑ Munyanziza, E.; Kehri, H. K.; Bagyaraj, D. J. (1997). "Agricultural intensification, soil biodiversity and agro-ecosystem function in the tropics: the role of mycorrhiza in crops and trees". Applied Soil Ecology. 6 (1): 77–85. doi:10.1016/S0929-1393(96)00152-7.
- 1 2 3 4 5 6 Amaranthus, Michael P. The importance and conservation of ectomycorrhizal fungal diversity in forest ecosystems: lessons from Europe and the Pacific Northwest. US Department of Agriculture, Forest Service, Pacific Northwest Research Station, 1998.
- ↑ Grant, Cynthia; et al. (2005). "Soil and fertilizer phosphorus: Effects on plant P supply and mycorrhizal development" (PDF). Canadian Journal of Plant Science. 85 (1): 3–14. doi:10.4141/p03-182.
- ↑ Scott, Neal A.; et al. (1999). "Soil carbon storage in plantation forests and pastures: land‐use change implications". Tellus B. 51 (2): 326–335. doi:10.1034/j.1600-0889.1999.00015.x.
- ↑ Chapela, Ignacio H.; et al. (2001). "Ectomycorrhizal fungi introduced with exotic pine plantations induce soil carbon depletion" (PDF). Soil Biology and Biochemistry. 33 (12): 1733–1740. doi:10.1016/s0038-0717(01)00098-0.
- 1 2 Kernaghan, G.; et al. (2002). "In Vitro Selection of Boreal Ectomycorrhizal Fungi for Use in Reclamation of Saline‐Alkaline Habitats" (PDF). Restoration Ecology. 10 (1): 43–51. doi:10.1046/j.1526-100x.2002.10105.x.
- 1 2 3 4 5 6 Colpaert, J (2011). "How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution". Annals of Forest Science. 68: 17–24. doi:10.1007/s13595-010-0003-9.
- ↑ Gadd, G.M. (1993). "Interaction of fungi with toxic metals". New Phytologist. 124: 25–60. doi:10.1111/j.1469-8137.1993.tb03796.x.
- 1 2 Blaudez, D.; Jacob, C.; Turnau, K.; Colpaert, J.V.; Ahonen-Jonnath, U.; Finlay, R.; Botton, B.; Chalot, M. (2000). "Differential responses of ectomycorrhizal fungi to heavy metals in vitro". Mycological Research. 104: 1366–1371. doi:10.1017/s0953756200003166.
- ↑ Gadd, G.M. (2004). "Microorganisms and heavy metal toxicity". Microbial Ecology. 4: 303–317. doi:10.1007/bf02013274.
- ↑ Bellion, M.; Courbot, M; Jacob, C.; et al. (2006). "Extracellular and cellular mechanism sustaining metal tolerance in ectomycorrhizal fungi". FEMS Microbiology Letters. 254: 173–181. doi:10.1111/j.1574-6968.2005.00044.x.
- ↑ Lasat, M.M.; Baker, A.J.M.; Kochian, L.V. (1998). "Altered Zn compartmentation in the root symplasm and stimulated Zn absorption into the leaf as mechanisms involved in Zn hyperaccumulation in Thlaspi caerulescens". Plant Physiol. 118: 875–883. doi:10.1104/pp.118.3.875.
- ↑ Leyval, C.; Turnau, K.; Haselwandter, K. (1997). "Effect of heavy metal pollution on mycorrhizal colonization and function: physiological, ecological and applied aspects". Mycorrhiza. 7 (3): 139–153. doi:10.1007/s005720050174.
- ↑ Southworth, D.; Tackleberry, L.E.; Massicotte, H.B. (2013). "Mycorrhizal ecology on serpentine soils". Plant Ecology and Diversity. 7: 445–455. doi:10.1080/17550874.2013.848950.
- ↑ Colpaert, J.V. 2008. Heavy metal pollution and genetic adaptations in ectomycorrhizal fungi. In: Avery S.V., Stratford M., Van West P. (eds) Stress in yeasts and filamentous fungi. Academic, Amsterdam, pp 157–174.
- ↑ Ruotsalainen, A.L.; Markkola, A.M.; Kozlov, M.V. (2009). "Mycorrhizal colonisation of mountain birch (Betula pubescens ssp czerepanovii) along three environmental gradients: does life in harsh environments alter plant–fungal relationships?". Environ Monit Assess. 148: 215–232. doi:10.1007/s10661-007-0152-y.
- ↑ Staudenrausch, S.; Kaldorf, M.; Renker, C.; Luis, P.; Buscot, F. (2005). "Diversity of the ectomycorrhiza community at a uranium mining heap". Biol Fertil Soils. 41: 439–446. doi:10.1007/s00374-005-0849-4.
- ↑ Branco, S.; Ree, R. (2010). "Serpentine soils do not limit mycorrhizal fungal diversity". PLOS ONE. 5: e11757. doi:10.1371/journal.pone.0011757.
- ↑ Egerton-Warburton, L.; Griffin, B. (1995). "Differential responses of Pisolithus tinctorius isolates to aluminium in vitro". Can J Bot. 73: 1229–1233. doi:10.1139/b95-133.
- ↑ Jourand, P.; Ducousso, M.; Loulergue-Majorel, C.; Hannibal, L.; Santoni, S.; Prin, Y.; Lebrun, M. (2010). "Ultramafic soils from New Caledonia structure Pisolithus albus in ecotype". FEMS Microbiology Ecology. 72: 238–249. doi:10.1111/j.1574-6941.2010.00843.x.
- 1 2 Colpaert, J.V.; Vandenkoornhuyse, P.; Adriaensen, K.; Vangronsveld, J. (2000). "Genetic variation and heavy metal tolerance in the ectomycorrhizal basidiomycete Suillus luteus". New Phytol. 147: 367–379. doi:10.1046/j.1469-8137.2000.00694.x.
- ↑ Colpaert, J.V.; Muller, L.A.H.; Lambaerts, M.; Adriaensen, K.; Vangronsveld, J. (2004). "Evolutionary adaptation to zinc toxicity in populations of Suilloid fungi". New Phytol. 162: 549–559. doi:10.1111/j.1469-8137.2004.01037.x.
- ↑ Krznaric, E.; Verbruggen, N.; Wevers, J.H.L.; Carleer, R.; Vangronsveld, J.; Colpaert, J.V. (2009). "Cd-tolerant Suillus luteus: a fungal insurance for pines exposed to Cd.". Environ Pollut. 157: 1581–1588. doi:10.1016/j.envpol.2008.12.030. PMID 19211178.
- ↑ Adriaensen, K.; Vrålstad, T.; Noben, J.P.; Vangronsveld, J.; Colpaert, J.V. (2005). "Copper-adapted Suillus luteus, a symbiotic solution for pines colonizing Cu mine spoils". Appl Environ Microbiol. 71: 7279–7284. doi:10.1128/aem.71.11.7279-7284.2005.
- ↑ Ruytinx, J.; Nguyen, H.; Van Hees, M.; De Beeck, O.; Vangronsveld, J.; Carleer, R.; Colpaert, J.V.; Adriaensen, K. (2013). "Zinc export results in adaptive zinc tolerance in the ectomycorrhizal basidiomycete Suillus bovinus". Metallomics. 5: 1225–1233. doi:10.1039/c3mt00061c.
- ↑ Luo, Zhi-Bin; et al. (2011). "The ectomycorrhizal fungus (Paxillus involutus) modulates leaf physiology of poplar towards improved salt tolerance". Environmental and Experimental Botany. 72 (2): 304–311. doi:10.1016/j.envexpbot.2011.04.008.
- ↑ Nikolova, Ivanka; Johanson, Karl J.; Dahlberg, Anders (1997). "Radiocaesium in fruitbodies and mycorrhizae in ectomycorrhizal fungi". Journal of Environmental Radioactivity. 37 (1): 115–125. doi:10.1016/S0265-931X(96)00038-0.
- 1 2 Meharg, Andrew A.; Cairney, John WG (2000). "Ectomycorrhizas—extending the capabilities of rhizosphere remediation?." (PDF). Soil Biology and Biochemistry. 32 (11): 1475–1484. doi:10.1016/s0038-0717(00)00076-6.
- ↑ Joner, Erik J.; Leyval, Corinne; Colpaert, Jan V. (2006). "Ectomycorrhizas impede phytoremediation of polycyclic aromatic hydrocarbons (PAHs) both within and beyond the rhizosphere". Environmental Pollution. 142 (1): 34–38. doi:10.1016/j.envpol.2005.09.007.
- ↑ Fransson, Petra MA; Taylor, Andy FS; Finlay, Roger D. (2005). "Mycelial production, spread and root colonisation by the ectomycorrhizal fungi Hebeloma crustuliniforme and Paxillus involutus under elevated atmospheric CO2". Mycorrhiza. 15 (1): 25–31. doi:10.1007/s00572-003-0289-7.
- ↑ Garcia, Maria O.; et al. (2008). "Mycorrhizal dynamics under elevated CO2 and nitrogen fertilization in a warm temperate forest" (PDF). Plant and Soil. 303 (1-2): 301–310. doi:10.1007/s11104-007-9509-9.
- 1 2 Compant, Stéphane; Marcel; Der Heijden, GA Van; Sessitsch, Angela (2010). "Climate change effects on beneficial plant–microorganism interactions". FEMS Microbiology Ecology. 73 (2): 197–214. doi:10.1111/j.1574-6941.2010.00900.x.
- ↑ Malcolm, Glenna M.; et al. (2008). "Acclimation to temperature and temperature sensitivity of metabolism by ectomycorrhizal fungi". Global Change Biology. 14 (5): 1169–1180. doi:10.1111/j.1365-2486.2008.01555.x.
- ↑ Arnolds, E. E. F. (1991). "Decline of ectomycorrhizal fungi in Europe". Agriculture, Ecosystems & Environment. 35 (2): 209–244. doi:10.1016/0167-8809(91)90052-y.
- 1 2 Wiensczyk, Alan M., et al. "Ectomycorrhizae and forestry in British Columbia: A summary of current research and conservation strategies." Journal of Ecosystems and Management 2.1 (2002). PDF
- ↑ Amaranthus, Michael P., et al. Soil compaction and organic matter affect conifer seedling nonmycorrhizal and ectomycorrhizal root tip abundance and diversity. Forest Service research paper. No. PB--97-104301/XAB; FSRP-PNW--494. Forest Service, Portland, OR (United States). Pacific Northwest Research Station, 1996.
- ↑ Dahlberg, Anders; et al. (2001). "Post-fire legacy of ectomycorrhizal fungal communities in the Swedish boreal forest in relation to fire severity and logging intensity". Biological Conservation. 100 (2): 151–161. doi:10.1016/s0006-3207(00)00230-5.
- ↑ Mah, Karen; et al. (2001). "The impacts of broadcast burning after clear-cutting on the diversity of ectomycorrhizal fungi associated with hybrid spruce seedlings in central British Columbia" (PDF). Canadian Journal of Forest Research. 31 (2): 224–235. doi:10.1139/x00-158.
- ↑ Hawksworth, David L (1991). "The fungal dimension of biodiversity: magnitude, significance, and conservation". Mycological Research. 95 (6): 641–655. doi:10.1016/S0953-7562(09)80810-1.
External links
- Mycorrhizal Associations: The Web Resource Comprehensive illustrations and lists of mycorrhizal and nonmycorrhizal plants and fungi
- Mycorrhizas – a successful symbiosis Biosafety research into genetically modified barley
- International Mycorrhiza Society International Mycorrhiza Society
- MycorWiki a portal concerned with the biology and ecology of ectomycorrhizal fungi and other forest fungi.