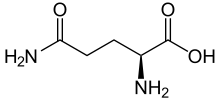
Glutamine
 L-Glutamine | |
 | |
| Names | |
|---|---|
| IUPAC name
Glutamine | |
| Other names
L-Glutamine (levo)glutamide 2-Amino-4-carbamoylbutanoic acid | |
| Identifiers | |
| 56-85-9 | |
| 3D model (Jmol) | Interactive image |
| Abbreviations | Gln, Q |
| ChEBI | CHEBI:28300 |
| ChEMBL | ChEMBL930 |
| ChemSpider | 718 |
| ECHA InfoCard | 100.000.266 |
| EC Number | 200-292-1 |
| 723 | |
| KEGG | C00303 |
| PubChem | 738 |
| UNII | 0RH81L854J |
| |
| |
| Properties[1] | |
| C5H10N2O3 | |
| Molar mass | 146.15 g·mol−1 |
| Melting point | decomposes around 185°C |
| soluble | |
| Acidity (pKa) | 2.2 (carboxyl), 9.1 (amino) |
| Chiral rotation ([α]D) |
+6.5º (H2O, c = 2) |
| Pharmacology | |
| A16AA03 (WHO) | |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Glutamine (abbreviated as Gln or Q; encoded by the codons CAA and CAG) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain amide which replaces the side chain hydroxyl of glutamic acid with an amine functional group, classifying it as a charge neutral, polar (at physiological pH) amino acid. It is non-essential and conditionally essential in humans, meaning the body can usually synthesize sufficient amounts of it, but in some instances of stress, the body's demand for glutamine increases and glutamine must be obtained from the diet.[2][3]
In human blood, glutamine is the most abundant free amino acid, with a concentration of about 500–900 µM.[4]
Functions
Glutamine plays a role in a variety of biochemical functions:
- Protein synthesis, as any other of the 20 proteinogenic amino acids
- Lipid synthesis, especially by cancer cells.[5][6]
- Regulation of acid-base balance in the kidney by producing ammonium[7]
- Cellular energy, as a source, next to glucose[8]
- Nitrogen donation for many anabolic processes, including the synthesis of purines[4]
- Carbon donation, as a source, refilling the citric acid cycle[9]
- Nontoxic transporter of ammonia in the blood circulation
- Precursor to the neurotransmitter glutamate
Producing and consuming organs
Producers
Glutamine is synthesized by the enzyme glutamine synthetase from glutamate and ammonia. The most relevant glutamine-producing tissue is the muscle mass, accounting for about 90% of all glutamine synthesized. Glutamine is also released, in small amounts, by the lung and the brain.[10] Although the liver is capable of relevant glutamine synthesis, its role in glutamine metabolism is more regulatory than producing, since the liver takes up large amounts of glutamine derived from the gut.[4]
Consumers
The most eager consumers of glutamine are the cells of intestines,[4] the kidney cells for the acid-base balance, activated immune cells,[11] and many cancer cells.[5][6][9]
Medical uses
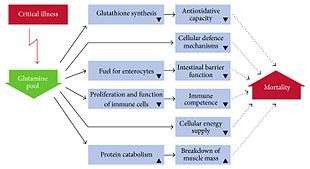
In catabolic states of injury and illness, glutamine becomes conditionally essential requiring intake from food or supplements.[13]
Glutamine has been used as a component of oral supplementation to reverse cachexia (muscle wasting) in patients with advanced cancer[14] or HIV/AIDS.[15]
Glutamine oral supplementation significantly reduces the risk of systemic infections originating from the gut such as in critically ill individuals and in individuals who have had abdominal surgery. The reduction in rates of infections in these groups of people are due to glutamine improving intestinal barrier function including reducing increased intestinal permeability. Intravenous administration does not appear to produce these benefits, however.[16]
Supplementation does not appear to have an effect in infants with significant problems of the stomach or intestines.[17]
Structure
Nutrition
Occurrences in nature
Glutamine is the most abundant naturally occurring, nonessential amino acid in the human body, and one of the few amino acids that can directly cross the blood–brain barrier.[18] In the body, it is found circulating in the blood, as well as stored in the skeletal muscles. It becomes conditionally essential (requiring intake from food or supplements) in states of illness or injury.[13]
Dietary sources
Dietary sources of L-glutamine include beef, pork, chicken, fish, eggs, milk, dairy products, wheat, cabbage, beets, beans, spinach, and parsley. Small amounts of free L-glutamine are also found in vegetable juices.[13]
See also
References
- ↑ Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, FL: CRC Press. p. C-311. ISBN 0-8493-0462-8..
- ↑ Dietary Reference Intakes: The Essential Guide to Nutrient Requirements, published by the Institute of Medicine's Food and Nutrition Board, currently available online at http://fnic.nal.usda.gov/dietary-guidance/dietary-reference-intakes/dri-reports
- ↑ Lacey, JM; Wilmore, DW (Aug 1990). "Is glutamine a conditionally essential amino acid?". Nutrition Reviews. 48 (8): 297–309. doi:10.1111/j.1753-4887.1990.tb02967.x.
- 1 2 3 4 Brosnan, John T. (June 2003). "Interorgan amino acid transport and its regulation". J. Nutr. 133 (6 Suppl 1): 2068S–2072S. PMID 12771367.

- 1 2 Corbet C, Feron O (2015). "Metabolic and mind shifts: from glucose to glutamine and acetate addictions in cancer". Current Opinion in Clinical Nutrition and Metabolic Care. 18 (4): 346–353. doi:10.1097/MCO.0000000000000178. PMID 26001655.
- 1 2 Gouw AM, Toal GG, Felsher DW (2016). "Metabolic vulnerabilities of MYC-induced cancer". Oncotarget. doi:10.18632/oncotarget.7223. PMID 26863454.
- ↑ Hall, John E.; Guyton, Arthur C. (2006). Textbook of medical physiology (11th ed.). St. Louis, Mo: Elsevier Saunders. p. 393. ISBN 0-7216-0240-1.
- ↑ Aledo, J. C. (2004). "Glutamine breakdown in rapidly dividing cells: Waste or investment?". BioEssays. 26 (7): 778–785. doi:10.1002/bies.20063. PMID 15221859.
- 1 2 Yuneva, M.; Zamboni, N.; Oefner, P.; Sachidanandam, R.; Lazebnik, Y. (2007). "Deficiency in glutamine but not glucose induces MYC-dependent apoptosis in human cells". The Journal of Cell Biology. 178 (1): 93–105. doi:10.1083/jcb.200703099. PMC 2064426
 . PMID 17606868.
. PMID 17606868. - ↑ Newsholme, P.; Lima, M. M. R.; Procopio, J.; Pithon-Curi, T. C.; Doi, S. Q.; Bazotte, R. B.; Curi, R. (2003). "Glutamine and glutamate as vital metabolites". Brazilian Journal of Medical and Biological Research. 36 (2): 153–163. doi:10.1590/S0100-879X2003000200002. PMID 12563517.
- ↑ Newsholme, P. (2001). "Why is L-glutamine metabolism important to cells of the immune system in health, postinjury, surgery or infection?". The Journal of Nutrition. 131 (9 Suppl): 2515S–2522S; discussion 2522S–4S. PMID 11533304.
- ↑ Stehle P, Kuhn KS (2015). "Glutamine: an obligatory parenteral nutrition substrate in critical care therapy". Biomed Res Int. 2015: 545467. doi:10.1155/2015/545467. PMC 4606408
 . PMID 26495301.
. PMID 26495301. - 1 2 3 "Glutamine". Medical Reference Guide. University of Maryland Medical Center. 2011-05-24. Archived from the original on 2013-12-09. Retrieved 2014-03-03.
- ↑ May, PE; Barber, A; D'Olimpio, JT; Hourihane, A; Abumrad, NN (April 2002). "Reversal of cancer-related wasting using oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine, and glutamine.". American journal of surgery. 183 (4): 471–9. doi:10.1016/s0002-9610(02)00823-1. PMID 11975938.
- ↑ "Glutamine". WebMD. WebMD, LLC. Retrieved 2015-03-15.
- ↑ Rapin JR, Wiernsperger N (2010). "Possible links between intestinal permeability and food processing: A potential therapeutic niche for glutamine". Clinics (Sao Paulo). 65 (6): 635–43. doi:10.1590/S1807-59322010000600012. PMC 2898551
 . PMID 20613941.
. PMID 20613941. - ↑ Brown, JV; Moe-Byrne, T; McGuire, W (15 December 2014). "Glutamine supplementation for young infants with severe gastrointestinal disease.". The Cochrane database of systematic reviews. 12: CD005947. doi:10.1002/14651858.CD005947.pub4. PMID 25504522.
- ↑ Lee, W. J.; Hawkins, R. A.; Viña, J. R.; Peterson, D. R. (1998). "Glutamine transport by the blood-brain barrier: A possible mechanism for nitrogen removal". The American journal of physiology. 274 (4 Pt 1): C1101–C1107. PMID 9580550.
