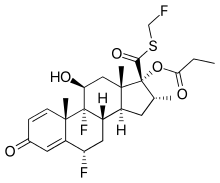
Fluticasone propionate
 | |
| Clinical data | |
|---|---|
| Trade names | Flixotide (inhalatory), Flixonase (nasal) |
| AHFS/Drugs.com | Monograph |
| Pregnancy category |
|
| Routes of administration | Intranasal, Inhaled, Topical Cream or Ointment |
| ATC code | D07AC17 (WHO) & D07AC04 (WHO) (topical) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 0.51% (Intranasal) |
| Protein binding | 91% |
| Metabolism |
Intranasal Hepatic (CYP3A4-mediated) |
| Biological half-life | 10 hours |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number |
80474-14-2 |
| PubChem (CID) | 444036 |
| IUPHAR/BPS | 7080 |
| DrugBank |
DB00588 |
| ChemSpider |
392059 |
| UNII |
O2GMZ0LF5W |
| ChEBI |
CHEBI:31441 |
| ChEMBL |
CHEMBL1473 |
| ECHA InfoCard | 100.129.097 |
| Chemical and physical data | |
| Formula | C25H31F3O5S |
| Molar mass | 500.57 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Fluticasone propionate belongs to a class of drugs known as corticosteroids, specifically glucocorticoids, which are hormones that predominantly affect the metabolism of carbohydrates and, to a lesser extent, fat and protein. It is used to treat asthma, allergic rhinitis, nasal polyps, various skin disorders and Crohn's disease and ulcerative colitis. It is also used to treat eosinophilic esophagitis.
Medical uses
Asthma
Fluticasone is used by powder or aerosol inhalation for the prophylaxis of asthma. Typical initial doses in the UK range from 100 to 250 micrograms twice daily in mild asthma up to 1 mg twice daily in severe asthma, adjusted according to response. Children over four years of age may be given initial doses of 50 to 100 micrograms twice daily, increased to 200 micrograms twice daily if necessary. The drug may also be given via a nebuliser in severe chronic asthma. Usual adult doses are 0.5 to 2 mg twice daily. Children aged four to sixteen years may be given 1 mg twice daily.
Allergic rhinitis
Nasal spray preparation of fluticasone propionate is used in the prophylaxis and treatment of allergic rhinitis. The usual dose is 100 micrograms into each nostril once daily, increased if necessary to 100 micrograms into each nostril twice daily. Children aged 4-11 years may be given half these doses.
Nasal polyps
Fluticasone propionate nasal drops are used in the treatment of nasal polyps. 200 micrograms should be instilled into each nostril once or twice daily for at least four to six weeks.
Other
Creams and ointments containing 0.05% and 0.005% Fluticasone propionate, respectively, are available and applied topically in the treatment of various skin disorders. It can be given orally in the treatment of Crohn’s disease and ulcerative colitis. Some benefit was also reported in coeliac disease. The dose is 5 mg four times daily but some consider higher doses necessary.
Adverse effects
If taken correctly, the nasal spray and oral inhaler formulation have less corticosteroid side effects than the tablet formulation because they limit systemic (blood) absorption.[1] However, if the spray or inhaler is used at higher than recommended doses or with other corticosteroids, serious side effects can occur.[1][2] These systemic corticosteroid side effects include weakened immune system, increased risk of systemic infections, osteoporosis, and elevated pressure in the eyes.[3]
Nasal spray
Common side effects may include nasal irritation, headache, nausea, vomiting, diarrhea, nosebleed, and cough. Rare side effects include painful white patches in nose or throat, sore throat, bruising (erythema nodosum), vision problems, swelling of face or neck, and difficulty breathing or swallowing.[4]
Oral inhaler
Common side effects may include upper respiratory tract infection, throat irritation, thrush, cough, and headache. Rare side effects include bruising, swelling of the face/neck, depression, tiredness, and shortness of breath.[5]
Pharmacology
Fluticasone propionate is a highly selective agonist at the glucocorticoid receptor with negligible activity at androgen, estrogen, or mineralocorticoid receptors, thereby producing anti-inflammatory and vasoconstriction effects. It has been shown to have a wide range of inhibitory effects on multiple cell types (e.g. mast cell, eosinophil, neutrophil, macrophages, and lymphocytes) and mediators (e.g. histamine, eicosanoids, leukotrienes, and cytokines) involved in inflammation. Fluticasone propionate is stated to exert a topical effect on the lungs without significant systemic effects at usual doses, due to its low systemic bioavailability.
Interactions
Fluticasone propionate is broken down by CYP3A4 (Cytochrome P450 3A4), and has been shown to interact with strong CYP3A4 inhibitors such as ritonavir and ketoconazole.[1][2]
Ritonavir is a common drug used in the treatment of HIV. Coadministration of ritonavir and fluticasone may lead to increased levels of fluticasone in the body, which may lead to Cushing’s Syndrome and adrenal suppression.[6]
Ketoconazole, an antifungal drug, has also been shown to increase fluticasone concentration leading to systemic corticosteroid side effects.[1][2]
See also
References
- 1 2 3 4 Flonase[package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2003.
- 1 2 3 Flovent[package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014
- ↑ http://www.mayoclinic.org/steroids/art-20045692?pg=2
- ↑ AHFS Consumer Medication Information [Internet]. Bethesda (MD): American Society of Health-System Pharmacists, Inc.; ©2008. Fluticasone Nasal Spray; [revised 2010 Sept 1; reviewed 2010 Sept 1; cited 2014 Nov 2]; [about 1 p.]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a695002.html
- ↑ AHFS Consumer Medication Information [Internet]. Bethesda (MD): American Society of Health-System Pharmacists, Inc.; ©2008. Fluticasone Oral Inhalation; [revised 2010 Sept 1; reviewed 2010 Sept 1; cited 2014 Nov 2]; [about 1 p.]. Available from: http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601056.html
- ↑ Foisy, M., Yakiwchuk, E., Chiu, I. and Singh, A. (2008), Adrenal suppression and Cushing's syndrome secondary to an interaction between ritonavir and fluticasone: a review of the literature. HIV Medicine, 9: 389–396. doi: 10.1111/j.1468-1293.2008.00579.x