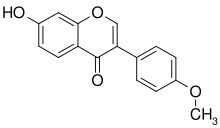
Formononetin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
7-hydroxy-3-(4-methoxyphenyl)chromen-4-one | |
| Other names
Biochanin B Formononetol 7-Hydroxy-4'-methoxyisoflavone 4'-O-methyldaidzein | |
| Identifiers | |
| 485-72-3 | |
| 3D model (Jmol) | Interactive image |
| 237979 | |
| ChEBI | CHEBI:18088 |
| ChEMBL | ChEMBL242341 |
| ChemSpider | 4444070 |
| ECHA InfoCard | 100.006.931 |
| KEGG | C00858 |
| PubChem | 5280378 |
| |
| |
| Properties | |
| C16H12O4 | |
| Molar mass | 268.26 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Formononetin is an O-methylated isoflavone.
Natural occurrences
Formononetin is found in a number of plants and herbs like the red clover.[1] Along with other phytoestrogens, it predominantly occurs in leguminous plants and Fabaceae, particularly in beans, such as green beans, lima beans, soy and many others, as the free aglycone or in form of its glucoside ononin.[2]
It can also be found in Maackia amurensis cell cultures.[3]
Metabolism
The enzyme 4'-methoxyisoflavone 2'-hydroxylase uses formononetin, NADPH, H+, and O2 to produce 2'-hydroxyformononetin, NADP+ and H2O.
The enzyme Isoflavone 3'-hydroxylase uses formononetin, NADPH, H+, and O2 to produce calycosin (3'-hydroxyformononetin), NADP+ and H2O.
Glycosides
Ononin is the 7-O-glucoside of formononetin.
References
- ↑ Medjakovic, S.; Jungbauer, A. (2008). "Red Clover Isoflavones Biochanin A and Formononetin are Potent Ligands of the Human Aryl Hydrocarbon Receptor". The Journal of Steroid Biochemistry and Molecular Biology. 108 (1–2): 171–177. doi:10.1016/j.jsbmb.2007.10.001. PMID 18060767.
- ↑ "Iowa State University Database on the Isoflavone Content of Foods, Release 1.3". USDA. 2002.
- ↑ Isoflavonoid production by callus cultures of Maackia amurensis. S.A Fedoreyev, T.V Pokushalov, M.V Veselova, L.I Glebko, N.I Kulesh, T.I Muzarok, L.D Seletskaya, V.P Bulgakov and Yu.N Zhuravlev, Fitoterapia, 1 August 2000, Volume 71, Issue 4, Pages 365–372, doi:10.1016/S0367-326X(00)00129-5
This article is issued from Wikipedia - version of the 5/30/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.