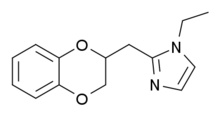
Imiloxan
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
81167-16-0 |
| PubChem (CID) | 133621 |
| IUPHAR/BPS | 3470 |
| ChemSpider |
117866 |
| UNII |
ZJ56PS1DWK |
| ChEMBL |
CHEMBL578481 |
| Chemical and physical data | |
| Formula | C14H16N2O2 |
| Molar mass | 244.289 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Imiloxan is a drug which is used in scientific research. It acts as a selective antagonist for the α2B adrenergic receptor,[1] and has been useful for distinguishing the actions of the different α2 adrenergic subtypes.[2][3]
Synthesis
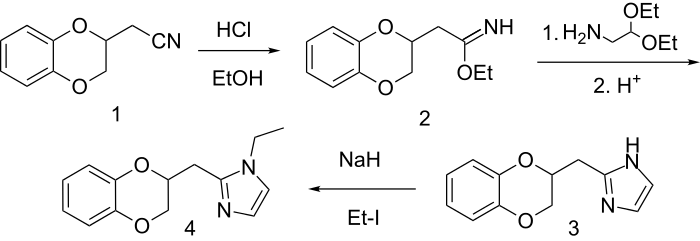
Imiloxan Synthesis:[4]
The imidazole portion of imiloxan is prepared by the reaction of an imidate with the diethyl acetal of aminoacetaldehyde. N-Alkylation of the imidazole with ethyl iodide gives imiloxan.
References
- ↑ Michel AD, Loury DN, Whiting RL (March 1990). "Assessment of imiloxan as a selective alpha 2B-adrenoceptor antagonist". British Journal of Pharmacology. 99 (3): 560–4. doi:10.1111/j.1476-5381.1990.tb12968.x. PMC 1917331
 . PMID 1970500.
. PMID 1970500. - ↑ Cobos-Puc LE, Villalón CM, Sánchez-López A, Lozano-Cuenca J, Pertz HH, Görnemann T, Centurión D (January 2007). "Pharmacological evidence that alpha2A- and alpha2C-adrenoceptors mediate the inhibition of cardioaccelerator sympathetic outflow in pithed rats". European Journal of Pharmacology. 554 (2–3): 205–11. doi:10.1016/j.ejphar.2006.09.068. PMID 17109851.
- ↑ Romero TR, de Castro Perez A, de Francischi JN, Gama Duarte ID (April 2009). "Probable involvement of alpha(2C)-adrenoceptor subtype and endogenous opioid peptides in the peripheral antinociceptive effect induced by xylazine". European Journal of Pharmacology. 608 (1–3): 23–7. doi:10.1016/j.ejphar.2009.02.019. PMID 19236861.
- ↑ Caroon, Joan M.; Clark, Robin D.; Kluge, Arthur F.; Olah, Ronald; Repke, David B.; Unger, Stefan H.; Michel, Anton D.; Whiting, Roger L. (1982). "Structure-activity relationships for 2-substituted imidazoles as .alpha.2-adrenoceptor antagonists". Journal of Medicinal Chemistry. 25 (6): 666–70. doi:10.1021/jm00348a012. PMID 6124635.
This article is issued from Wikipedia - version of the 5/31/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.