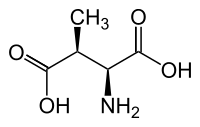
L-threo-3-Methylaspartate
 | |
| Names | |
|---|---|
| IUPAC name
(2S,3S)-2-Amino-3-methylbutanedioic acid | |
| Other names
L-threo-3-Methylaspartate; 3-Methylaspartic acid | |
| Identifiers | |
| 6061-13-8 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:47980 |
| ChemSpider | 389071 |
| KEGG | C03618 |
| PubChem | 440064 |
| |
| |
| Properties | |
| C5H9NO4 | |
| Molar mass | 147.13 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
L-threo-3-Methylaspartate is an unusual amino acid formed by glutamate mutase and can be metabolised by methylaspartate ammonia-lyase. It is found in the structures of the antibiotics friulimicin[1] and vicenistatin[1] and in carbon metabolism of haloarchaea (Methylaspartate cycle).[2]
References
- 1 2 Ogasawara, Y.; Kakinuma, K.; Eguchi, T. (2005). "Involvement of Glutamate Mutase in the Biosynthesis of the Unique Starter Unit of the Macrolactam Polyketide Antibiotic Vicenistatin". The Journal of Antibiotics. 58 (7): 468–72. doi:10.1038/ja.2005.62. PMID 16161486.
- ↑ Khomyakova, M.; Bukmez, O.; Thomas, L. K.; Erb, T. J.; Berg, I. A. (2011). "A Methylaspartate Cycle in Haloarchaea". Science. 331 (6015): 334–7. doi:10.1126/science.1196544. PMID 21252347.
This article is issued from Wikipedia - version of the 9/4/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.