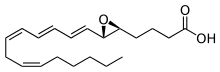
Leukotriene A4
 | |
| Names | |
|---|---|
| IUPAC name
4-{(2S,3S)-3-[(1E,3E,5Z,8Z)-1,3,5,8-Tetradecatetraen-1-yl]-2-oxiranyl}butanoic acid | |
| Systematic IUPAC name
4-{(2S,3S)-3-[(1E,3E,5Z,8Z)-1,3,5,8-Tetradecatetraen-1-yl]-2-oxiranyl}butanoic acid | |
| Identifiers | |
| 72059-45-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15651 |
| ChemSpider | 4444074 |
| 5214 | |
| KEGG | C00909 |
| MeSH | D017572 |
| PubChem | 5280383 |
| |
| |
| Properties | |
| C20H30O3 | |
| Molar mass | 318.450 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Leukotriene A4 is a leukotriene. Leukotriene A4 hydrolase converts it to leukotriene B4. Leukotriene C4 synthase converts it to leukotriene C4.

Eicosanoid synthesis. (Leukotrienes at right.)
References
This article is issued from Wikipedia - version of the 11/30/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.