Lipid II
 | |
| Identifiers | |
|---|---|
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27692 |
| ChemSpider | 26333143 |
| PubChem | 46173749 |
| |
| |
| Properties | |
| C94H156N8O26P2 | |
| Molar mass | 1876.23 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
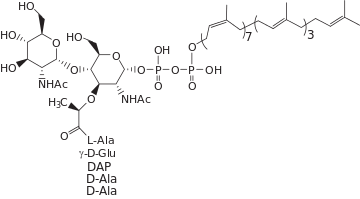
Lipid II is a precursor molecule in the synthesis of the cell wall of bacteria. It is a peptidoglycan, which is amphipathic and named for its bactoprenol hydrocarbon chain, which acts as a lipid anchor, embedding itself in the bacterial cell membrane. Lipid II must translocate across the cell membrane to deliver and incorporate its disaccharide-pentapeptide "building block" into the peptidoglycan mesh. Lipid II is the target of several antibiotics.
Synthesis
In peptidoglycan biosynthetic pathway
Lipid II is the final intermediate in peptidoglycan synthesis. It is formed when the MurG transferase catalyzes addition of N-acetylglucosamine (GlcNAc) to Lipid I, resulting in a complete disaccharide-pentapeptide monomer with a bactoprenol-pyrophosphate anchor. This occurs on the inside of the cytoplasmic membrane, where the bactoprenol chain is embedded in the inner leaflet of the bilayer. Lipid II is then flipped across the membrane to expose the disaccharide-pentapeptide monomer, which is the pentapeptide stem consisting of L-Ala-γ-D-Glu-m-DAP-D-Ala-D-Ala between GlcNAc and N-acetylmuramic acid (MurNAc), for polymerization and cross-linking into peptidoglycan. The remaining bactoprenol-pyrophosphate is then recycled to the interior of the membrane. Lipid II has been referred to as the "shuttle carrier" of peptidoglycan "building blocks'.[1]
The essential flippase that translocates lipid II across the cytoplasmic membrane was only published in July 2014, after decades of searching.[2]
Artificial production
A method for artificial production of lipid II has been described. For synthesis of lipid II from UDP-MurNAc pentapeptide and undecaprenol, the enzymes MraY, MurG, and undecaprenol kinase can be used.[3] Synthetic Lipid II analogues are used in experiments studying how it interacts with and binds molecules.[4]
Functions
Polymers of lipid II form a linear glycan chain. This reaction is catalyzed by the glycosyltransferases of family 51 (GT51). Transpeptidases cross link the chains and form a net-like peptidoglycan macromolecule. The resulting glycopeptide is essential part of the envelope of many bacteria. Lipid II was estimated to exist at a concentration of less than 2000 molecules per bacterial cell.[5]
Lipid II biosynthesis is functional and essential even in organisms without a cell wall like Chlamydia and Wolbachia. It has been hypothesized that maintaining lipid II biosynthesis reflects its role in prokaryotic cell division.[6]
In the discovery and mechanism of assembly of pili in gram positive bacteria Lipid II has been implicated as a crucial structural molecule. It anchors the pili during or after polymerization of the pilus components.[7]
Antibiotics
Since Lipid II must be flipped outside the cytoplasmic membrane before incorporation of its disaccharide-peptide unit into peptidoglycan, it is a relatively accessible target for antibiotics. These antibiotics fight bacteria by either directly inhibiting the peptidoglycan synthesis, or by binding to lipid II to form destructive pores in the cytoplasmic membrane.[8] Examples of antibiotics that target Lipid II include:
- Vancomycin and its synthetic derivatives[8]
- Ramoplanin[8]
- Several lantibiotics, including the common food preservative nisin[9]
- Teixobactin[10]
- Copsin
- Human alpha defensins
Binding
The D-Ala-D-Ala terminus is used by glycopeptide antibiotic vancomycin to inhibit lipid I- and lipid II-consuming peptidoglycan synthesis; in vancomycin-resistant strains vancomycin cannot bind, because a crucial hydrogen bond is lost. Oritavancin also uses the D-Ala-D-Ala terminus, but in addition it uses the crossbridge and D-iso-glutamine in position 2 of the lipid II stem peptide, as present in a number of Gram-positive pathogens, like staphylococci and enterococci. The increased binding of oritavancin through amidation of lipid II can compensate for the loss of a crucial hydrogen bond in vancomycin-resistant strains,[11]
Lantibiotics recognize lipid-II by its pyrophosphate.[1]
Lipid II interacts with human alpha defensins, a class of antimicrobial peptides, such as Defensin, alpha 1. The latter has been used to describe and predict binding of synthetic low-molecular weight compounds created as possible therapeutic agents in treating of Gram-positive infections.[12]
Penicillin-binding protein 4 exchanges d-amino acids into Lipid II (and Lipid I), acting as a transpeptidase in vitro.[13]
References
- 1 2 Anton Chugunov; Darya Pyrkova; Dmitry Nolde; Anton Polyansky; Vladimir Pentkovsky; Roman Efremov (Apr 16, 2013). "Lipid-II forms potential "landing terrain" for lantibiotics in simulated bacterial membrane". Sci Rep. 3: 1678. doi:10.1038/srep01678. PMC 3627190
 . PMID 23588060.
. PMID 23588060. - ↑ Lok-To Sham; Emily K. Butler; Matthew D. Lebar; et al. (11 July 2014). "MurJ is the flippase of lipid-linked precursors for peptidoglycan biogenesis". Science. 345 (6193): 220–222. doi:10.1126/science.1254522.
- ↑ Huang LY, Huang SH, Chang YC, Cheng WC, Cheng TJ, Wong CH (28 July 2014). "Enzymatic synthesis of lipid II and analogues.". Angew Chem Int Ed Engl. 53 (31): 8060–5. doi:10.1002/anie.201402313.
- ↑ t Hart P, Oppedijk S, Breukink E, Martin NI (2016). "New Insights into Nisin's Antibacterial Mechanism Revealed by Binding Studies with Synthetic Lipid II Analogues.". Biochemistry. 55 (1): 232–7. doi:10.1021/acs.biochem.5b01173.
- ↑ Y. van Heijenoort; M. Gomez; M. Derrien; J. Ayala; J. van Heijenoort (1992). "Membrane intermediates in the peptidoglycan metabolism of Escherichia coli: possible roles of PBP 1b and PBP 3.". J. Bacteriol. 174: 3549–3557;.
- ↑ Henrichfreise B, Schiefer A, Schneider T, et al. (September 2009). "Functional conservation of the lipid II biosynthesis pathway in the cell wall-less bacteria Chlamydia and Wolbachia: why is lipid II needed?". Mol Microbiol. 73 (5): 913–23. doi:10.1111/j.1365-2958.2009.06815.x.
- ↑ Pili in Gram-positive pathogens, Nature, vol 4, pg 513
- 1 2 3 Heijenoort J (December 2007). "Lipid Intermediates in the Biosynthesis of Bacterial Peptidoglycan". Microbiol Mol Biol Rev. 71: 620–635. doi:10.1128/MMBR.00016-07. Retrieved 13 January 2015.
- ↑ de Kruijff B, van Dam V, Breukink E (12 November 2008). "Lipid II: A central component in bacterial cell wall synthesis and a target for antibiotics". Prostaglandins Leukot Essent Fatty Acids. 79: 117–21. doi:10.1016/j.plefa.2008.09.020. PMID 19008088.
- ↑ Wright, Gerard (7 January 2015). "Antibiotics: An irresistible newcomer". Nature. 517: 442–444. doi:10.1038/nature14193.
- ↑ Münch D, Engels I, Müller A, Reder-Christ K, Falkenstein-Paul H, Bierbaum G, Grein F, Bendas G, Sahl HG, Schneider T (17 November 2014). "Structural variations of the cell wall precursor lipid II - Influence on binding and activity of the lipoglycopeptide antibiotic oritavancin". Antimicrobial Agents and Chemotherapy. 59: 772–781. doi:10.1128/AAC.02663-14. Retrieved 10 January 2015.
- ↑ Varney KM, Bonvin AM, Pazgier M, et al. (2013). "Turning defense into offense: defensin mimetics as novel antibiotics targeting lipid II.". PLoS Pathog. 9 (11): e1003732. doi:10.1371/journal.ppat.1003732. PMC 3820767
 . PMID 24244161.
. PMID 24244161. - ↑ Qiao Y, Lebar MD, Schirner K, Schaefer K, Tsukamoto H, Kahne D, Walker S (22 October 2014). "Detection of lipid-linked peptidoglycan precursors by exploiting an unexpected transpeptidase reaction.". J Am Chem Soc. 136 (42): 14678–81. doi:10.1021/ja508147s.
External links
- Lipid II at the US National Library of Medicine Medical Subject Headings (MeSH)