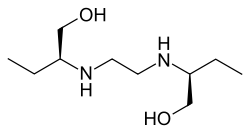
Ethambutol
 | |
 | |
 | |
| Names | |
|---|---|
| Other names
(2S,2’S)-2,2’-(Ethane-1,2-diyldiimino)dibutan-1-ol[1] | |
| Identifiers | |
| 74-55-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:4877 |
| ChEMBL | ChEMBL44884 |
| ChemSpider | 13433 |
| DrugBank | DB00330 |
| ECHA InfoCard | 100.000.737 |
| EC Number | 200-810-26 |
| KEGG | D07925 |
| MeSH | Ethambutol |
| PubChem | 14052 |
| UNII | 8G167061QZ |
| |
| |
| Properties | |
| C10H24N2O2 | |
| Molar mass | 204.31 g·mol−1 |
| Appearance | White crystals |
| Odor | Odourless |
| log P | −0.291 |
| Pharmacology | |
| J04AK02 (WHO) | |
| |
| Oral | |
| Pharmacokinetics: | |
| 20–30% | |
| Hepatic | |
| 3–4 hours | |
| Renal | |
| Related compounds | |
| Related compounds |
|
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ethambutol (commonly abbreviated EMB or simply E) is a medication primarily used to treat tuberculosis.[2] It is usually given in combination with other tuberculosis drugs, such as isoniazid, rifampicin and pyrazinamide. It may also be used to treat Mycobacterium avium complex, and Mycobacterium kansasii.[3]
It can cause problems with vision, liver problems and allergies among other side effects.[3] It is pregnancy category C in the United States due to concerns with eye issues and category A in Australia meaning that they did not find evidence of harm after being taken by many pregnant women.[3][4] It is reasonable to use during breast feeding if required.[3]
It is on the World Health Organization's List of Essential Medicines, a list of the most important medication needed in a basic health system.[5] It is sold under the trade names Myambutol and Servambutol.
Medical uses
Ethambutol is used along with other medications to treat a number of infections including: tuberculosis, Mycobacterium avium complex, and Mycobacterium kansasii.[3]
Adverse effects
- Optic neuritis[6] (hence contraindicated in children below six years of age)
- Red-green color blindness[7]
- Peripheral neuropathy
- Arthralgia
- Hepatotoxicity
- Hyperuricaemia
- Vertical nystagmus
- Milk skin reaction
Mechanism of action
Ethambutol is bacteriostatic against actively growing TB bacilli. It works by obstructing the formation of cell wall. Mycolic acids attach to the 5'-hydroxyl groups of D-arabinose residues of arabinogalactan and form mycolyl-arabinogalactan-peptidoglycan complex in the cell wall. It disrupts arabinogalactan synthesis by inhibiting the enzyme arabinosyl transferase. Disruption of the arabinogalactan synthesis inhibits the formation of this complex and leads to increased permeability of the cell wall.
Pharmacokinetics
It is well absorbed from the gastrointestinal tract and well distributed in body tissues and fluids. 50% is excreted unchanged in urine.
References
- ↑ "ethambutol (CHEBI:4877)". Chemical Entities of Biological Interest. UK: European Bioinformatics Institute. 18 August 2010. Main. Retrieved 26 April 2012.
- ↑ Yendapally R, Lee RE (March 2008). "Design, synthesis, and evaluation of novel ethambutol analogues". Bioorg. Med. Chem. Lett. 18 (5): 1607–11. doi:10.1016/j.bmcl.2008.01.065. PMC 2276401
 . PMID 18242089.
. PMID 18242089. - 1 2 3 4 5 "Ethambutol Hydrochloride". The American Society of Health-System Pharmacists. Retrieved 24 April 2014.
- ↑ "Prescribing medicines in pregnancy database". Australian Government. 3 March 2014. Retrieved 22 April 2014.
- ↑ "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Retrieved 22 April 2014.
- ↑ Lim SA (April 2006). "Ethambutol-associated optic neuropathy" (PDF). Ann. Acad. Med. Singap. 35 (4): 274–8. PMID 16710500.
- ↑ Tripathi, K D (August 2015). Essentials of MEDICAL PHARMACOLOGY (Seventh ed.). India: JAYPEE BROTHERS MEDICAL PUBLISHERS. p. 769. ISBN 978-93-5025-937-5.