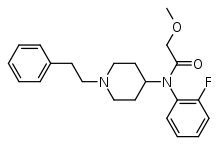
Ocfentanil
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| Synonyms | Ocfentanyl, A-3217 |
| CAS Number |
101343-69-5 |
| PubChem (CID) | 60575 |
| ChemSpider |
54604 |
| UNII |
MX52WBC8EV |
| Chemical and physical data | |
| Formula | C22H27FN2O2 |
| Molar mass | 370.460 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Ocfentanil (INN) (also called A-3217) is a potent synthetic opioid structurally related to fentanyl[1] that was developed in the early 1990s as one of a series of potent naloxone-reversible opioids in an attempt to obtain an opioid that had better therapeutic indices in terms of cardiovascular effects and respiratory depression as compared to fentanyl.[2]
Study of the analgesic activity of ocfentanil using the mouse hot plate test (55 °C) gave an ED50 of 0.007 mg/kg compared to 0.018 mg/kg for fentanyl; ocfentanil being approximately 2.5 times as potent as fentanyl in this test.[3]
In human volunteers ocfentanil induces effective analgesia at 1 μg/kg, while in doses up to 3 μg/kg, analgesia and respiratory depression occurred in a dose-dependent manner. While a further study suggests that ocfentanil may be as effective as morphine in post-operative relief,[4] Ocfentanil was also studied as a supplement to general anaesthesia, in which the researchers concluded that it appears to be similar in action to fentanyl, with 3 μg/kg of ocfentanil approximately equivalent to 5 μg/kg of fentanyl.[5][6]
Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[7][8]
Legal Status
As of October 2015 Ocfentanil is a controlled substance in China.[9]
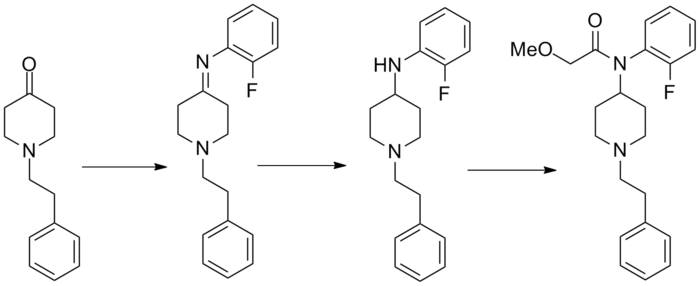
Synthesis
References
- ↑ Filer, C. N.; Nugent, R. P.; Huang, B. S. (November 1995). "The synthesis of [fluorophenyl-3H(N)] ocfentanil and [fluorophenyl-3H(N)] brifentanil". Journal of Labelled Compounds and Radiopharmaceuticals. 36 (11): 1019–1027. doi:10.1002/jlcr.2580361102. ISSN 1099-1344.
- ↑ US Patent application 2002/176888 A1
- ↑ Bagley, Jerome R.; Kudzma, Linas V.; Lalinde, Nhora L.; Colapret, John A.; Huang, Bao-Shang; Lin, Bor-Sheng; Jerussi, Thomas P.; Benvenga, Mark J.; Doorley, Brian M.; Ossipov, Michael H.; Spaulding, Theodore C.; Spencer, H. Kenneth; Rudo, Frieda G.; Wynn, Richard L. (July 1991). "Evolution of the 4-anilidopiperidine class of opioid analgesics". Medicinal Research Reviews. 11 (4): 403–436. doi:10.1002/med.2610110404. ISSN 1098-1128. PMID 1875771.
- ↑ Glass, P.; Camporesi, E. M.; Martel, D.; Afifi, M. S. "The Analgesic Efficacy of A3217". Anesthesiology. 71 (Supplement). doi:10.1097/00000542-198909001-00321.
- ↑ Fletcher, James E.; Sebel, Peter s.; Murphy, Michael R.; Mick, Stephan A.; Fein, Seymour (November 1991). "Comparison of Ocfentanil and Fentanyl as Supplements to General Anesthesia". Anesthesia & Analgesia. 73 (5). doi:10.1213/00000539-199111000-00019. PMID 1952145.
- ↑ Ebrahim Z (1991). "Multiple dose evaluation of the efficacy of ocfentanil hydrochloride (A3217) to produce postoperative analgesia.". Anesthesia and Analgesia. 72.
- ↑ Mounteney, Jane; Giraudon, Isabelle; Denissov, Gleb; Griffiths, Paul (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe". International Journal of Drug Policy. 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
- ↑ Dussy, F. E.; Hangartner, S.; Hamberg, C.; Berchtold, C.; Scherer, U.; Schlotterbeck, G.; Wyler, D.; Briellmann, T. A. (2016). "An Acute Ocfentanil Fatality: A Case Report with Postmortem Concentrations". Journal of Analytical Toxicology. doi:10.1093/jat/bkw096. ISSN 1945-2403.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.