Dapiprazole
Not to be confused with Aripiprazole.
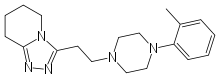 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Consumer Drug Information |
| MedlinePlus | a601043 |
| Pregnancy category |
|
| Routes of administration | Topical (eye drops) |
| ATC code | S01EX02 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Negligible when administered topically |
| Identifiers | |
| |
| CAS Number |
72822-12-9 |
| PubChem (CID) | 3033538 |
| IUPHAR/BPS | 7155 |
| DrugBank |
DB00298 |
| ChemSpider |
2298190 |
| UNII |
5RNZ8GJO7K |
| KEGG |
D07775 |
| ChEBI |
CHEBI:51066 |
| ChEMBL |
CHEMBL1201216 |
| Chemical and physical data | |
| Formula | C19H27N5 |
| Molar mass | 325.451 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Dapiprazole (Rev-Eyes) is an alpha blocker. It is used to reverse mydriasis after eye examination.[1]
References
- ↑ Doughty, Michael J.; Lyle, William M. (May 1992). "A Review of the Clinical Pharmacokinetics of Pilocarpine, Moxisylyte (Thymoxamine), and Dapiprazole in the Reversal of Diagnostic Pupillary Dilation". Optometry & Vision Science. 69 (5).
This article is issued from Wikipedia - version of the 5/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.