Trirhenium nonachloride
 | |
| Names | |
|---|---|
| IUPAC name
Rhenium(III) chloride | |
| Other names
Rhenium trichloride | |
| Identifiers | |
| 13569-63-6 | |
| 3D model (Jmol) | Interactive image |
| ECHA InfoCard | 100.033.610 |
| EC Number | 236-987-1 |
| PubChem | 83581 |
| |
| |
| Properties | |
| ReCl3 | |
| Molar mass | 292.57 g/mol |
| Appearance | red, crystalline, nonvolatile solid |
| Density | 4800 kg/m3 |
| Melting point | N/A |
| Boiling point | 500 °C (932 °F; 773 K) (decomposes) |
| hydrolyzes to form Re2O3.xH2O. | |
| Structure | |
| Rhombohedral, hR72 | |
| R-3m, No. 166 | |
| (trimeric solid and in solution) (dimeric in acetic acid) | |
| Hazards | |
| Main hazards | Corrosive (C) |
| Safety data sheet | External MSDS |
| Related compounds | |
| Other anions |
Rhenium tribromide Rhenium triiodide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Trirhenium nonachloride (Re3Cl9) is a compound of rhenium and chlorine. It was first discovered in 1932 by Geilnann, Wriuce, and Biltz.[1]
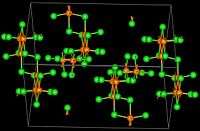
Structure and physical properties
The structure of trirhenium nonachloride consists of well-defined Re3Cl9 units that are connected by chloride bridges.[2]
Chemical properties
trirhenium nonachloride prepared from rhenium pentachloride without further treatment is chemically reactive, e.g. for producing adducts. If however the sample is vacuum sublimed at 500 °C, the resulting material comparatively unreactive. However, x-ray diffraction tests show no difference in structure between the untreated and vacuum-sublimed material.[2]
The heat of oxidation is evaluated according to the equation:
- 1/3 Re3Cl9 + 4 OH− + 2 OCl− → ReO4− + 2 H2O + 5Cl−
The enthalpy for this process is 190.7 ± 0.2 kcal/mol.[2]
Preparation
Trirhenium nonachloride is most efficiently prepared by thermal decomposition of rhenium pentachloride or hexachlororhenic(IV) acid. Other methods include treating rhenium with sulfuryl chloride. This process is sometimes conducted with the addition of aluminium chloride.[2] Heating Re2(O2CCH3)4Cl2 under HCl.[3]
- 3/2 Re2(O2CCH3)4Cl2 + 6 HCl → Re3Cl9 + 6 HO2CCH3
Uses
Trirhenium nonachloride is used as a starting material for synthesis of many rhenium complexes[4]
References
- ↑ Geilnann, W.; Wriuce, F. W.; Biltz. W.: Nachr. Ges. Wiss. Gottingen 1932, 579.
- 1 2 3 4 Colton, R. Chemistry of rhenium and technetium. 965.
- ↑ Lincoln R.; Wilkinson, G. (1980). "Trirhenium Nonachloride". Inorg. Synth. Inorganic Syntheses. 20: 44. doi:10.1002/9780470132517.ch12. ISBN 978-0-470-13251-7.
- ↑ Hamon, J-R, Astruc, D. (1989). "Organometallic electron reservoirs. 38. Influence of steric bulk on Fischer-type syntheses of peralkylated electron-reservoir sandwiches [FeCp*(arene)]+: cleavage of alkyl groups and mechanistic implications". Organometallics. 8 (9): 2243–2247. doi:10.1021/om00111a022.