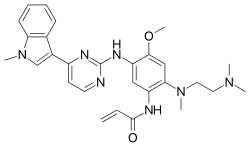
Osimertinib
 | |
| Clinical data | |
|---|---|
| Trade names | Tagrisso, Tagrix |
| AHFS/Drugs.com | tagrisso |
| Routes of administration | Oral tablets |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | Probably high[1] |
| Metabolism | Oxidation (CYP3A) |
| Biological half-life | 48 hours |
| Excretion | Feces (68%), urine (14%) |
| Identifiers | |
| |
| Synonyms | AZD9291 |
| CAS Number | 1421373-65-0 |
| PubChem (CID) | 71496458 |
| DrugBank | DB09330 |
| ChemSpider | 31042598 |
| UNII | 3C06JJ0Z2O |
| KEGG | D10766 |
| ChEBI |
CHEBI:90943 |
| Chemical and physical data | |
| Formula | C28H33N7O2 |
| Molar mass | 499.62 g·mol−1 |
| 3D model (Jmol) | Interactive image |
| |
| |
Osimertinib (previously known as mereletinib or AZD9291;[2] trade name Tagrisso) is a third-generation epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) drug[3][4] developed by AstraZeneca Pharmaceuticals – for mutated EGFR cancers.
Approvals and indications
In November 2015, after a Priority Review, the US FDA granted accelerated approval to osimertinib for the treatment of metastatic epidermal growth factor receptor (EGFR) T790M mutation-positive non-small cell lung cancer (NSCLC), as detected by an FDA-approved test, which has progressed on or after EGFR tyrosine kinase inhibitor (TKI) therapy.[5][6]
The FDA approval made reference to two clinical trials, in which an EGFR T790M mutation was confirmed by a Cobas EGFR mutation test; osimertinib was given as 80 mg once daily.[1][7]
The EU gave a similar approval in Feb 2016[8] after two Phase II studies (AURA extension and AURA2).
Clinical trials
First-line use
Osimertinib has also given encouraging results in early trials as a first-line therapy.[9]
References
- 1 2 "Tagrisso (osimertinib) Tablet, for Oral Use. Full Prescribing Information" (PDF). AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850. Retrieved 16 November 2015.
- ↑ "Proposed INN: List 113" (PDF). International Nonproprietary Names for Pharmaceutical Substances (INN). 29 (2): 285. 2015. Retrieved 16 November 2015.
- ↑ Ayeni D, Politi K, Goldberg SB (2015). "Emerging Agents and New Mutations in EGFR-Mutant Lung Cancer". Clin. Cancer Res. 21 (17): 3818–20. doi:10.1158/1078-0432.CCR-15-1211. PMID 26169963.
- ↑ Tan CS, Gilligan D, Pacey S (2015). "Treatment approaches for EGFR-inhibitor-resistant patients with non-small-cell lung cancer". Lancet Oncol. 16 (9): e447–59. doi:10.1016/S1470-2045(15)00246-6. PMID 26370354.
- ↑ U.S. Food and Drug Administration. Hematology/Oncology (Cancer) Approvals & Safety Notifications.
- ↑ Xu M, Xie Y, Ni S, Liu H (2015). "The latest therapeutic strategies after resistance to first generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR TKIs) in patients with non-small cell lung cancer (NSCLC)". Ann Transl Med. 3 (7): 96. doi:10.3978/j.issn.2305-5839.2015.03.60. PMC 4430733
 . PMID 26015938.
. PMID 26015938. - ↑ U.S. Food and Drug Administration. "Osimertinib".
- ↑ Tagrisso (osimertinib) approved in EU as first-in-class treatment for patients with EGFR T790M mutation-positive metastatic non-small cell lung cancer
- ↑ First-Line Osimertinib Yields Nearly 80% Response in Advanced Lung Cancer. April 2016